Chapter 17 Thermochemistry 17 1 The Flow of



























































- Slides: 190

Chapter 17 Thermochemistry 17. 1 The Flow of Energy 17. 2 Measuring and Expressing Enthalpy Changes 17. 3 Heat in Changes of State 17. 4 Calculating Heats of Reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Why does lava cool faster in water than in air? Lava flowing out of an erupting volcano is very hot. As lava flows, it loses heat and begins to cool slowly. The lava may flow into the ocean, where it cools more rapidly. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations What are the ways in which energy changes can occur? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations • Energy is the capacity for doing work or supplying heat. • Unlike matter, energy has neither mass nor volume. • Energy is detected only because of its effects. • Thermochemistry is the study of energy changes that occur during chemical reactions and changes in state. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations Every substance has a certain amount of energy stored inside it. • The energy stored in the chemical bonds of a substance is called chemical potential energy. • The kinds of atoms and the arrangement of the atoms in a substance determine the amount of energy stored in the substance. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations Every substance has a certain amount of energy stored inside it. • When you buy gasoline, you are actually buying the stored potential energy it contains. • The controlled explosions of the gasoline in a car’s engine transform the potential energy into useful work, which can be used to propel the car. • Heat is also produced, making the car’s engine extremely hot. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations Energy changes occur as either heat transfer or work, or a combination of both. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations Energy changes occur as either heat transfer or work, or a combination of both. • Heat, represented by q, is energy that transfers from one object to another because of a temperature difference between the objects. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Energy Transformations • Heat flows spontaneously from a warmer object to a cooler object. • If two objects remain in contact, heat will flow from the warmer object to the cooler object until the temperature of both objects is the same. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The energy released when a piece of wood is burned has been stored in the wood as A. sunlight. B. heat. C. calories. D. chemical potential energy. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The energy released when a piece of wood is burned has been stored in the wood as A. sunlight. B. heat. C. calories. D. chemical potential energy. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes What happens to the energy of the universe during a chemical or physical process? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes What happens to the energy of the universe during a chemical or physical process? • Chemical reactions and changes in physical state generally involve either the absorption or the release of heat. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes • You can define a system as the part of the universe on which you focus your attention. • Everything else in the universe makes up the surroundings. • Together, the system and its surroundings make up the universe. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes The law of conservation of energy states that in any chemical or physical process, energy is neither created nor destroyed. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes During any chemical or physical process, the energy of the universe remains unchanged. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes During any chemical or physical process, the energy of the universe remains unchanged. • If the energy of the system increases during that process, the energy of the surroundings must decrease by the same amount. • If the energy of the system decreases during that process, the energy of the surroundings must increase by the same amount. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes Direction of Heat Flow The direction of heat flow is given from the point of view of the system. • Heat is absorbed from the surroundings in an endothermic process. – Heat flowing into a system from its surroundings is defined as positive; q has a positive value. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes Direction of Heat Flow The direction of heat flow is given from the point of view of the system. • An exothermic process is one that releases heat to its surroundings. – Heat flowing out of a system into its surroundings is defined as negative; q has a negative value. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

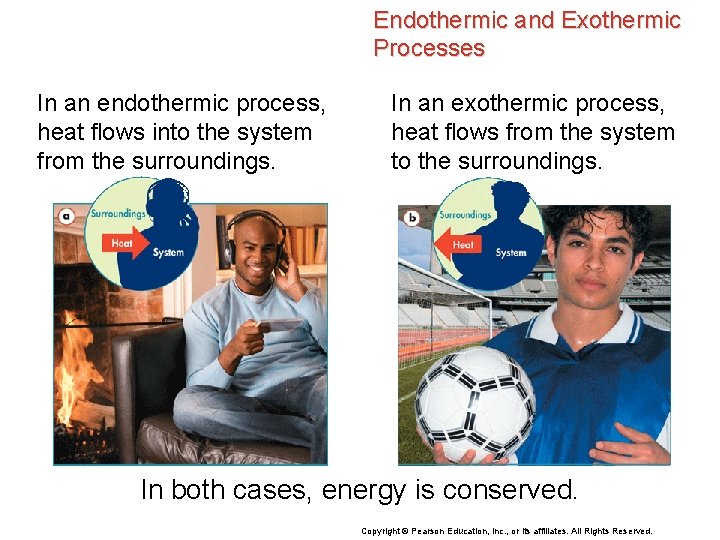
Endothermic and Exothermic Processes In an endothermic process, heat flows into the system from the surroundings. In an exothermic process, heat flows from the system to the surroundings. In both cases, energy is conserved. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 1 Recognizing Endothermic and Exothermic Processes On a sunny winter day, the snow on a rooftop begins to melt. As the melted water drips from the roof, it refreezes into icicles. Describe the direction of heat flow as the water freezes. Is this process endothermic or exothermic? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 1 1 Analyze Identify the relevant concepts. • Heat flows from a warmer object to a cooler object. • An endothermic process absorbs heat from the surroundings. • An exothermic process releases heat to the surroundings. First identify the system and surroundings. Then determine the direction of the heat flow. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 1 2 Solve Apply concepts to this situation. First identify the system and the surroundings. System: water Surroundings: air Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 1 2 Solve Apply concepts to this situation. Determine the direction of heat flow. • In order for water to freeze, its temperature must decrease. • Heat flows out of the water and into the air. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 1 2 Solve Apply concepts to this situation. Determine if the process is endothermic or exothermic. • Heat is released from the system to the surroundings. • The process is exothermic. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes Units for Measuring Heat Flow Heat flow is measured in two common units: • the calorie • the joule Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Endothermic and Exothermic Processes Units for Measuring Heat Flow A calorie (cal) is defined as the quantity of heat needed to raise the temperature of 1 g of pure water 1°C. • The word calorie is written with a small c except when referring to the energy contained in food. • The dietary Calorie is written with a capital C. • One dietary Calorie is equal to one kilocalorie, or 1000 calories. 1 Calorie = 1 kilocalorie = 1000 calories Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

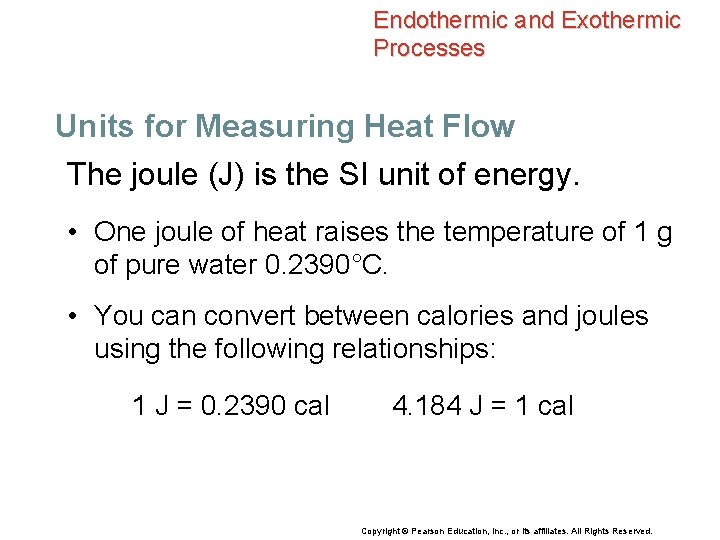
Endothermic and Exothermic Processes Units for Measuring Heat Flow The joule (J) is the SI unit of energy. • One joule of heat raises the temperature of 1 g of pure water 0. 2390°C. • You can convert between calories and joules using the following relationships: 1 J = 0. 2390 cal 4. 184 J = 1 cal Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Athletes often use instant cold packs to soothe injuries. Many of these packs use the dissociation of ammonium nitrate in water to create a cold-feeling compress. Is this reaction endothermic or exothermic? Why? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Athletes often use instant cold packs to soothe injuries. Many of these packs use the dissociation of ammonium nitrate in water to create a cold-feeling compress. Is this reaction endothermic or exothermic? Why? The instant cold pack feels cold because it removes heat from its surroundings. Therefore, the dissociation of ammonium nitrate in water is endothermic. The system (the cold pack) gains heat as the surroundings lose heat. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat On what factors does the heat capacity of an object depend? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat On what factors does the heat capacity of an object depend? • The amount of heat needed to increase the temperature of an object exactly 1 o. C is the heat capacity of that object. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat The heat capacity of an object depends on both its mass and its chemical composition. • The greater the mass of the object, the greater its heat capacity. • A massive steel cable requires more heat to raise its temperature by 1 o. C than a steel nail does. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

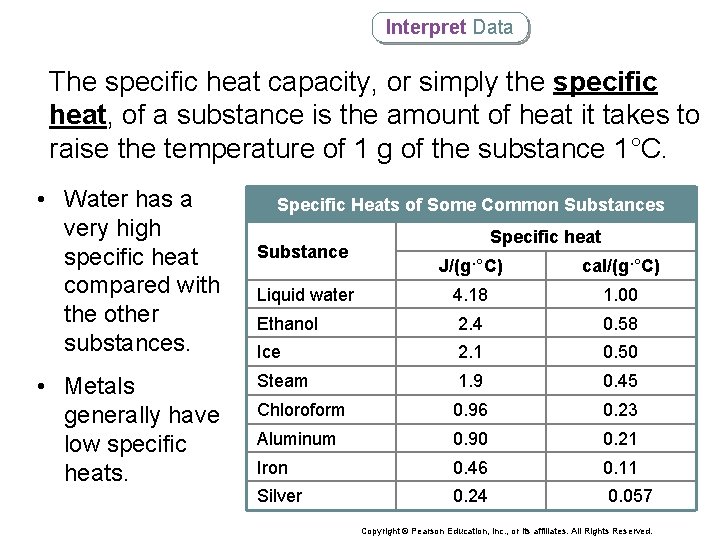
Interpret Data The specific heat capacity, or simply the specific heat, of a substance is the amount of heat it takes to raise the temperature of 1 g of the substance 1°C. • Water has a very high specific heat compared with the other substances. • Metals generally have low specific heats. Specific Heats of Some Common Substances Substance Specific heat J/(g·°C) cal/(g·°C) Liquid water 4. 18 1. 00 Ethanol 2. 4 0. 58 Ice 2. 1 0. 50 Steam 1. 9 0. 45 Chloroform 0. 96 0. 23 Aluminum 0. 90 0. 21 Iron 0. 46 0. 11 Silver 0. 24 0. 057 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

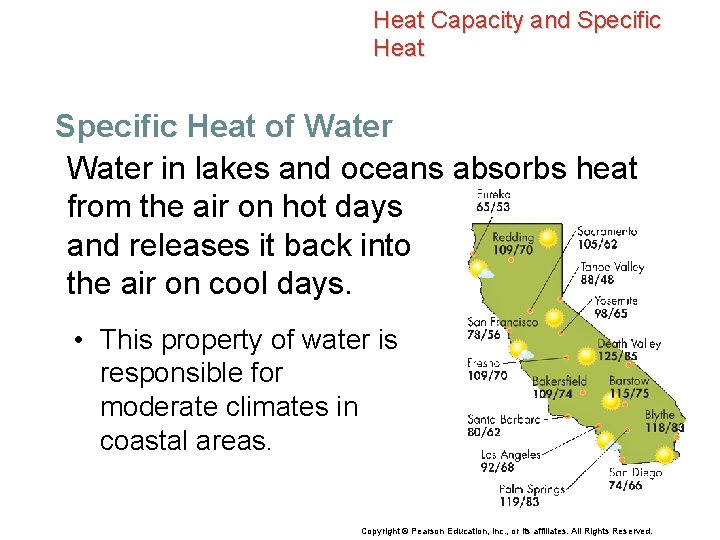
Heat Capacity and Specific Heat of Water Just as it takes a lot of heat to raise the temperature of water, water also releases a lot of heat as it cools. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat of Water in lakes and oceans absorbs heat from the air on hot days and releases it back into the air on cool days. • This property of water is responsible for moderate climates in coastal areas. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat of Water When a freshly baked apple pie comes out of the oven, both the filling and the crust are at the same temperature. • The filling, which is mostly water, has a higher specific heat than the crust. • In order to cool down, the filling must give off a lot of heat. • This release of heat is why you have to be careful not to burn your tongue when eating hot apple pie. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Heat will flow from the lava to the surroundings until the lava and surroundings are at the same temperature. Air has a smaller specific heat than water. Why would lava then cool more quickly in water than in air? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Heat will flow from the lava to the surroundings until the lava and surroundings are at the same temperature. Air has a smaller specific heat than water. Why would lava then cool more quickly in water than in air? Water requires more energy to raise its temperature than air. Therefore, lava in contact with water loses more heat energy than lava in contact with air, allowing it to cool more quickly. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

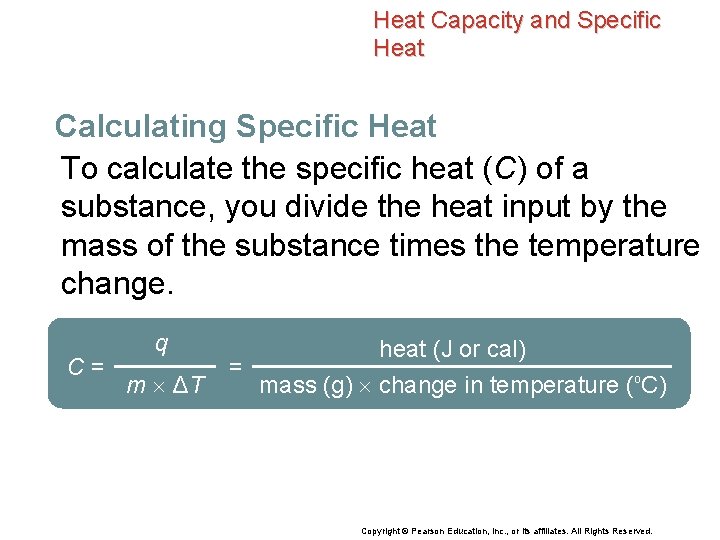
Heat Capacity and Specific Heat Calculating Specific Heat To calculate the specific heat (C) of a substance, you divide the heat input by the mass of the substance times the temperature change. C= q m ΔT heat (J or cal) = mass (g) change in temperature (o. C) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

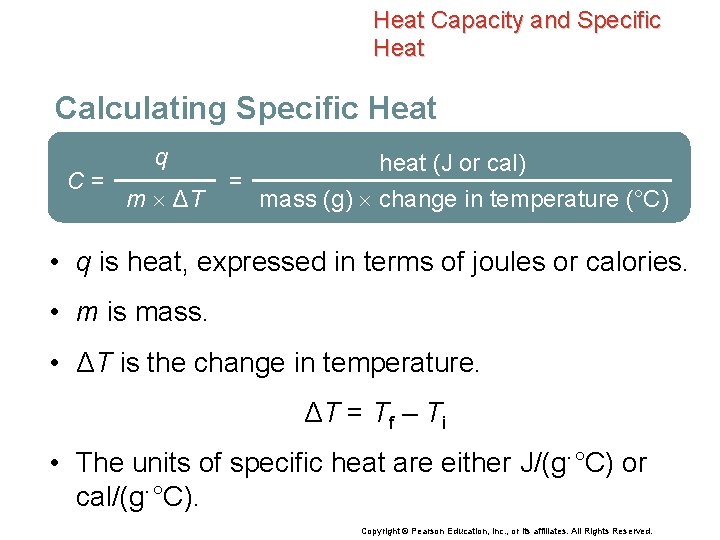
Heat Capacity and Specific Heat Calculating Specific Heat C= q m ΔT = heat (J or cal) mass (g) change in temperature (°C) • q is heat, expressed in terms of joules or calories. • m is mass. • ΔT is the change in temperature. ΔT = Tf – Ti • The units of specific heat are either J/(g·°C) or cal/(g·°C). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 2 Calculating the Specific Heat of a Substance The temperature of a 95. 4 -g piece of copper increases from 25. 0°C to 48. 0°C when the copper absorbs 849 J of heat. What is the specific heat of copper? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

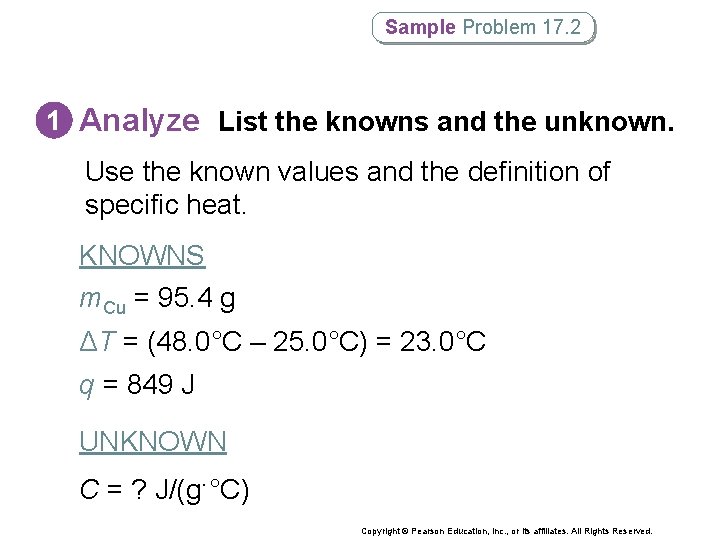
Sample Problem 17. 2 1 Analyze List the knowns and the unknown. Use the known values and the definition of specific heat. KNOWNS m. Cu = 95. 4 g ΔT = (48. 0°C – 25. 0°C) = 23. 0°C q = 849 J UNKNOWN C = ? J/(g·°C) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

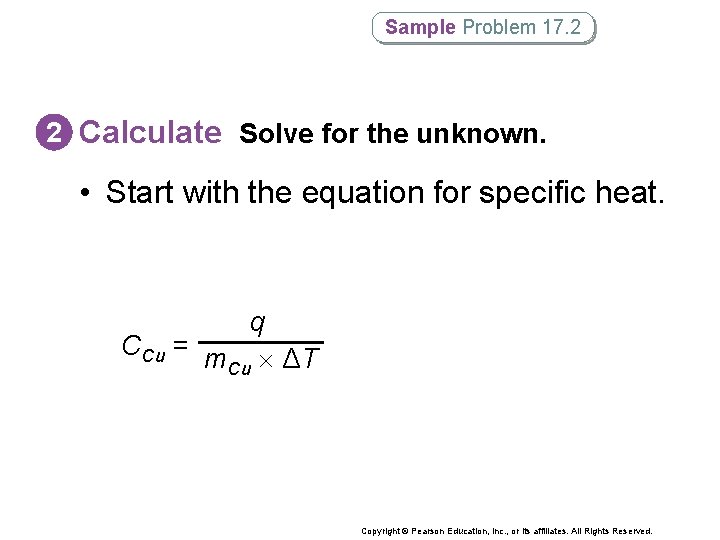
Sample Problem 17. 2 2 Calculate Solve for the unknown. • Start with the equation for specific heat. q CCu = m ΔT Cu Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

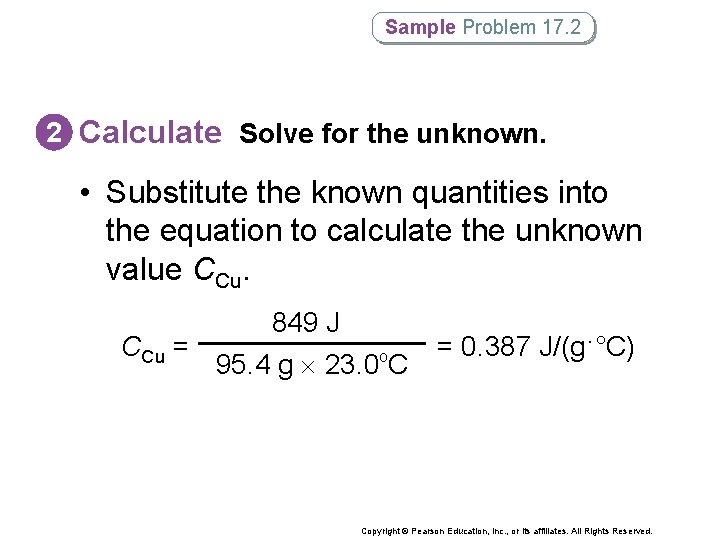
Sample Problem 17. 2 2 Calculate Solve for the unknown. • Substitute the known quantities into the equation to calculate the unknown value CCu = 849 J 95. 4 g 23. 0 C o = 0. 387 J/(g·°C) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 2 3 Evaluate Does the result make sense? • Remember that liquid water has a specific heat of 4. 18 J/(g·°C). • Metals have specific heats lower than water. • Thus, the calculated value of 0. 387 J/(g·°C) seems reasonable. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

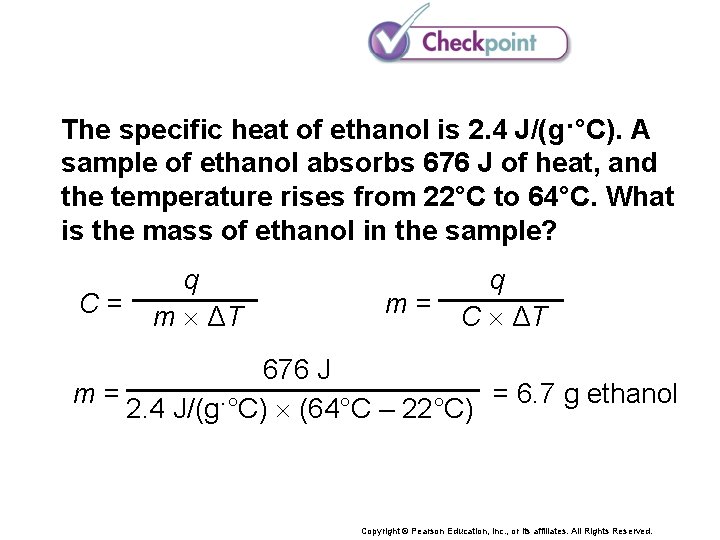
The specific heat of ethanol is 2. 4 J/(g·°C). A sample of ethanol absorbs 676 J of heat, and the temperature rises from 22°C to 64°C. What is the mass of ethanol in the sample? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The specific heat of ethanol is 2. 4 J/(g·°C). A sample of ethanol absorbs 676 J of heat, and the temperature rises from 22°C to 64°C. What is the mass of ethanol in the sample? C= q m ΔT q m = C ΔT 676 J m= = 6. 7 g ethanol 2. 4 J/(g·°C) (64°C – 22°C) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts & Key Equation Energy changes occur as either heat transfer or work, or a combination of both. During any chemical or physical process, the energy of the universe remains unchanged. The heat capacity of an object depends on both its mass and its chemical composition. C= q m ΔT Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • thermochemistry: the study of energy changes that occur during chemical reactions and changes in state • chemical potential energy: energy stored in chemical bonds • heat (q): energy that transfers from one object to another because of a temperature difference between the objects • system: a part of the universe on which you focus your attention • surroundings: everything in the universe outside the system Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • law of conservation of energy: in any chemical or physical process, energy is neither created nor destroyed • endothermic process: a process that absorbs heat from the surroundings • exothermic process: a process that releases heat to its surroundings • heat capacity: the amount of heat needed to increase the temperature of an object exactly 1°C • specific heat: the amount of heat needed to increase the temperature of 1 g of a substance 1°C; also called specific heat capacity Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

BIG IDEA Matter and Energy • During a chemical or physical process, the energy of the universe is conserved. – If energy is absorbed by the system in a chemical or physical process, the same amount of energy is released by the surroundings. – Conversely, if energy is released by the system, the same amount of energy is absorbed by the surroundings. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Chapter 17 Thermochemistry 17. 1 The Flow of Energy 17. 2 Measuring and Expressing Enthalpy Changes 17. 3 Heat in Changes of State 17. 4 Calculating Heats of Reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU How can you measure the amount of heat released when a match burns? Remember: The concept of specific heat allows you to measure heat flow in chemical and physical processes. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry How can you measure the change in enthalpy of a reaction? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry is the measurement of the heat flow into or out of a system for chemical and physical processes. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry is the measurement of the heat flow into or out of a system for chemical and physical processes. • In a calorimetry experiment involving an endothermic process, the heat absorbed by the system is equal to the heat released by its surroundings. • In an exothermic process, the heat released by the system is equal to the heat absorbed by its surroundings. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry is the measurement of the heat flow into or out of a system for chemical and physical processes. • The insulated device used to measure the absorption or release of heat in chemical or physical processes is called a calorimeter. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

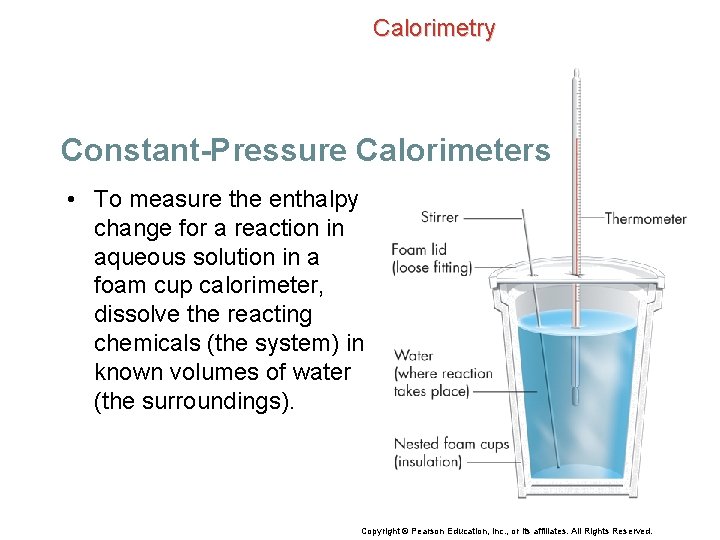
Calorimetry Constant-Pressure Calorimeters Foam cups can be used as simple calorimeters because they do not let much heat in or out. • Most chemical reactions and physical changes carried out in the laboratory are open to the atmosphere and thus occur at constant pressure. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Pressure Calorimeters The enthalpy (H) of a system accounts for the heat flow of the system at constant pressure. • The heat absorbed or released by a reaction at constant pressure is the same as the change in enthalpy, symbolized as ΔH. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Pressure Calorimeters The value of ΔH of a reaction can be determined by measuring the heat flow of the reaction at constant pressure. • In this textbook, the terms heat and enthalpy change are used interchangeably. • In other words, q = ΔH. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Pressure Calorimeters • To measure the enthalpy change for a reaction in aqueous solution in a foam cup calorimeter, dissolve the reacting chemicals (the system) in known volumes of water (the surroundings). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

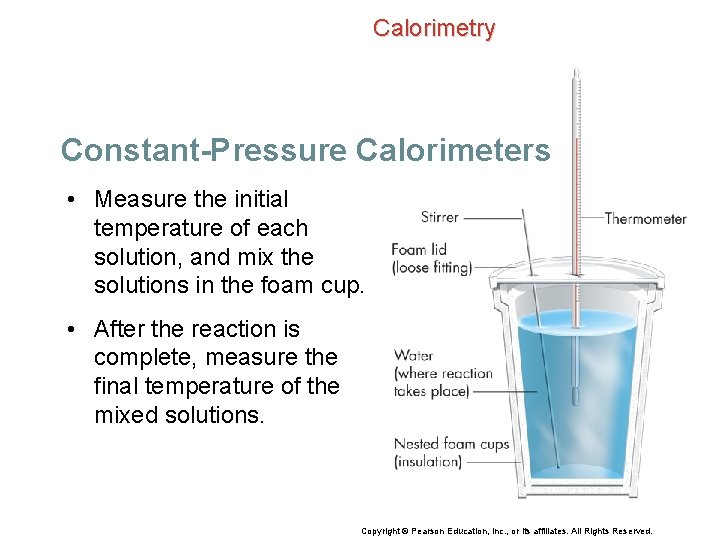
Calorimetry Constant-Pressure Calorimeters • Measure the initial temperature of each solution, and mix the solutions in the foam cup. • After the reaction is complete, measure the final temperature of the mixed solutions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

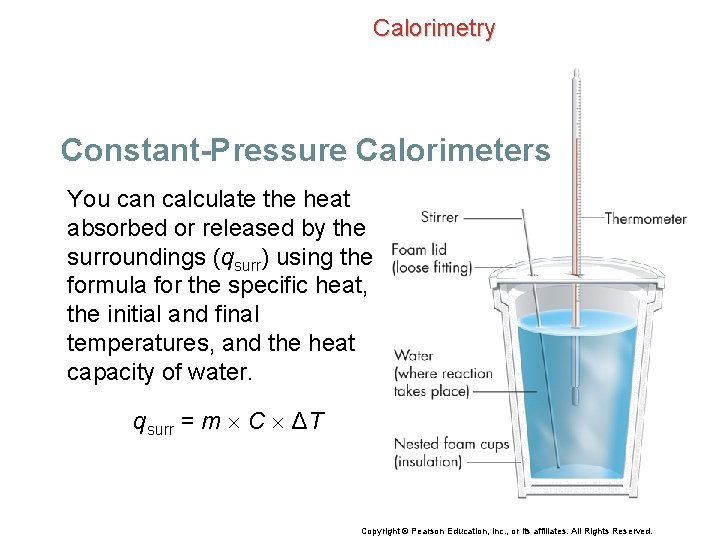
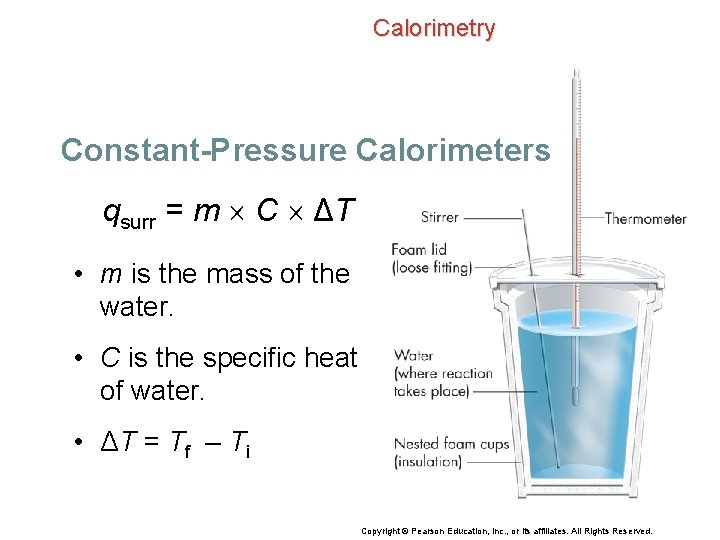
Calorimetry Constant-Pressure Calorimeters You can calculate the heat absorbed or released by the surroundings (qsurr) using the formula for the specific heat, the initial and final temperatures, and the heat capacity of water. qsurr = m C ΔT Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Pressure Calorimeters qsurr = m C ΔT • m is the mass of the water. • C is the specific heat of water. • ΔT = Tf – Ti Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Pressure Calorimeters The heat absorbed by the surroundings is equal to, but has the opposite sign of, the heat released by the system. qsurr = –qsys Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

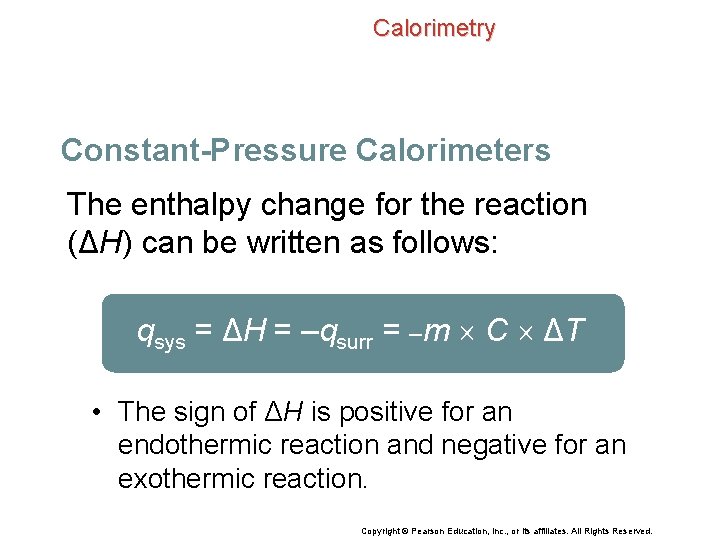
Calorimetry Constant-Pressure Calorimeters The enthalpy change for the reaction (ΔH) can be written as follows: qsys = ΔH = –qsurr = –m C ΔT • The sign of ΔH is positive for an endothermic reaction and negative for an exothermic reaction. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

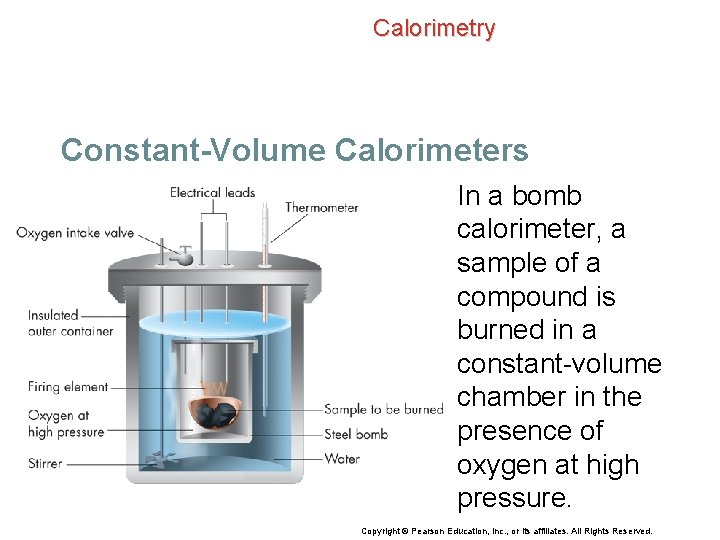
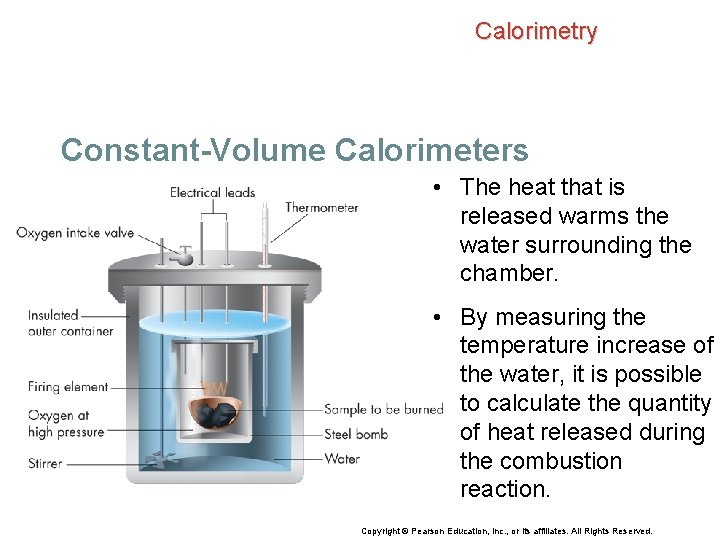
Calorimetry Constant-Volume Calorimeters Calorimetry experiments can also be performed at a constant volume using a device called a bomb calorimeter. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Volume Calorimeters In a bomb calorimeter, a sample of a compound is burned in a constant-volume chamber in the presence of oxygen at high pressure. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calorimetry Constant-Volume Calorimeters • The heat that is released warms the water surrounding the chamber. • By measuring the temperature increase of the water, it is possible to calculate the quantity of heat released during the combustion reaction. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What type of calorimeter would you use to measure the heat released when a match burns? Describe the experiment and how you would calculate the heat released. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What type of calorimeter would you use to measure the heat released when a match burns? Describe the experiment and how you would calculate the heat released. A constant-volume, or bomb, calorimeter would be used to measure the heat released when a match burns. The match would be ignited in the chamber. By measuring the temperature increase in the water and using the equation q = –m C ΔT , the heat released, q, can be calculated. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

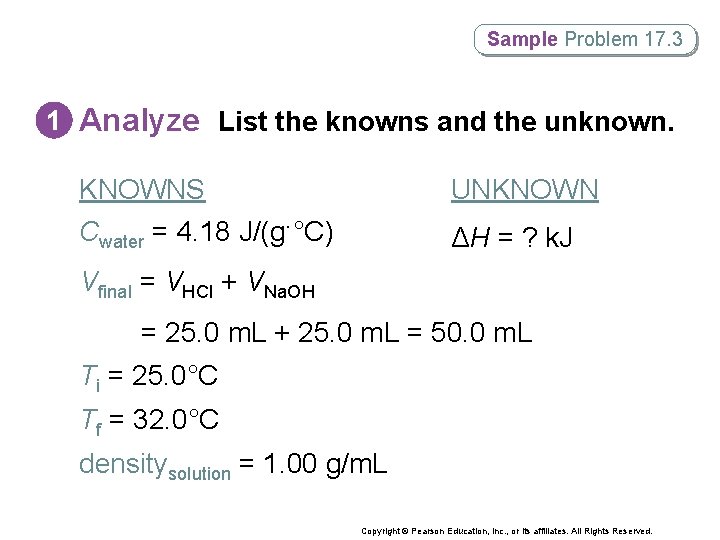
Sample Problem 17. 3 Enthalpy Change in a Calorimetry Experiment When 25. 0 m. L of water containing 0. 025 mol HCl at 25. 0°C is added to 25. 0 m. L of water containing 0. 025 mol Na. OH at 25. 0°C in a foam-cup calorimeter, a reaction occurs. Calculate the enthalpy change (in k. J) during this reaction if the highest temperature observed is 32. 0°C. Assume that the densities of the solutions are 1. 00 g/m. L and the volume of the final solution is equal to the sum of the volumes of the reacting solutions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 3 1 Analyze List the knowns and the unknown. • Use dimensional analysis to determine the mass of the water. • You must also calculate ΔT. • Use ΔH = –qsurr = –m C ΔT to solve for ΔH. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 3 1 Analyze List the knowns and the unknown. KNOWNS Cwater = 4. 18 J/(g·°C) UNKNOWN ΔH = ? k. J Vfinal = VHCl + VNa. OH = 25. 0 m. L + 25. 0 m. L = 50. 0 m. L Ti = 25. 0°C Tf = 32. 0°C densitysolution = 1. 00 g/m. L Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

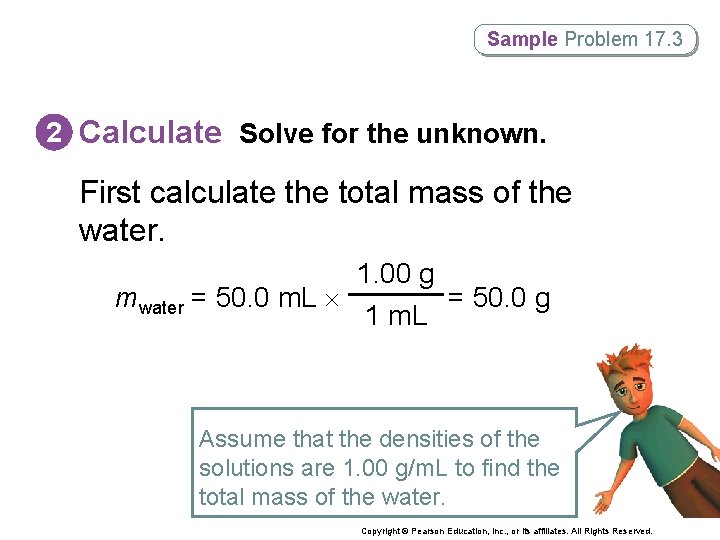
Sample Problem 17. 3 2 Calculate Solve for the unknown. First calculate the total mass of the water. 1. 00 g mwater = 50. 0 m. L = 50. 0 g 1 m. L Assume that the densities of the solutions are 1. 00 g/m. L to find the total mass of the water. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

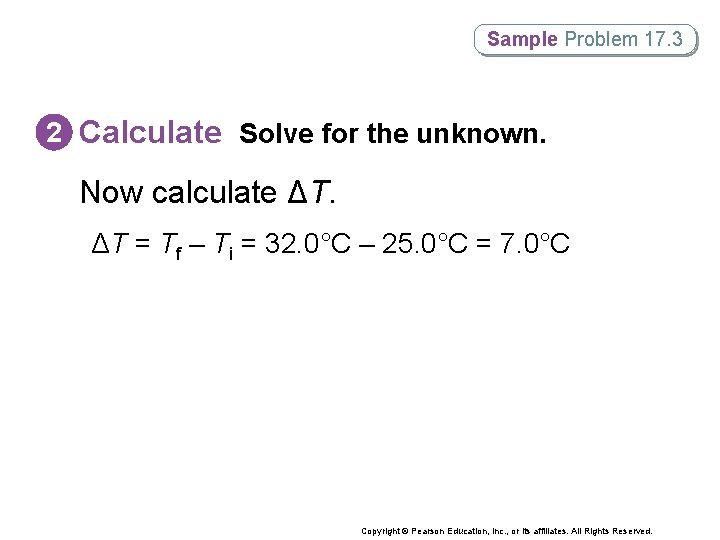
Sample Problem 17. 3 2 Calculate Solve for the unknown. Now calculate ΔT. ΔT = Tf – Ti = 32. 0°C – 25. 0°C = 7. 0°C Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

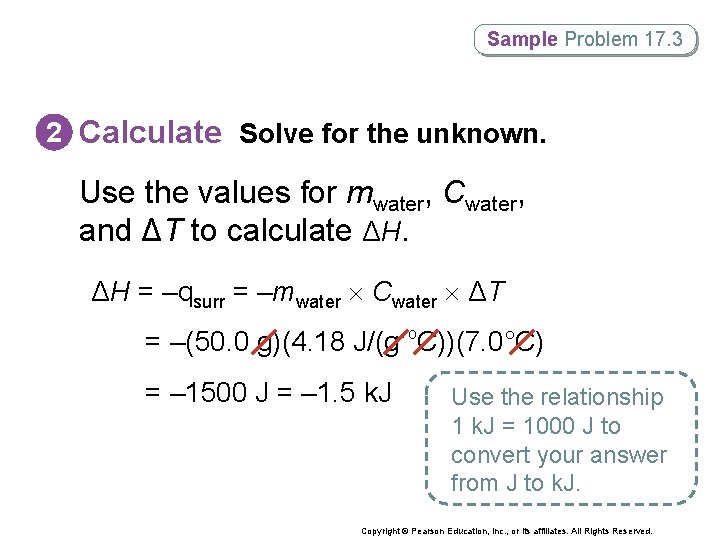
Sample Problem 17. 3 2 Calculate Solve for the unknown. Use the values for mwater, Cwater, and ΔT to calculate ΔH. ΔH = –qsurr = –mwater Cwater ΔT = –(50. 0 g)(4. 18 J/(g·o. C))(7. 0°C) = – 1500 J = – 1. 5 k. J Use the relationship 1 k. J = 1000 J to convert your answer from J to k. J. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 3 3 Evaluate Does the result make sense? • The temperature of the solution increases, which means that the reaction is exothermic, and thus the sign of ΔH should be negative. • About 4 J of heat raises the temperature of 1 g of water 1°C, so 200 J of heat is required to raise 50 g of water 1°C. Raising the temperature of 50 g of water 7°C requires about 1400 J, or 1. 4 k. J. • This estimated answer is very close to the calculated value of ΔH. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The initial temperature of the water in a constant-pressure calorimeter is 24°C. A reaction takes place in the calorimeter, and the temperature rises to 87°C. The calorimeter contains 367 g of water, which has a specific heat of 4. 18 J/(g·°C). Calculate the enthalpy change during this reaction. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The initial temperature of the water in a constant-pressure calorimeter is 24°C. A reaction takes place in the calorimeter, and the temperature rises to 87°C. The calorimeter contains 367 g of water, which has a specific heat of 4. 18 J/(g·°C). Calculate the enthalpy change during this reaction. ΔH = –m C ΔT = – 367 g 4. 18 J/(g·°C) (87°C – 24°C) = – 97000 J = – 97 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations How can you express the enthalpy change for a reaction in a chemical equation? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations In a chemical equation, the enthalpy change for the reaction can be written as either a reactant or a product. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

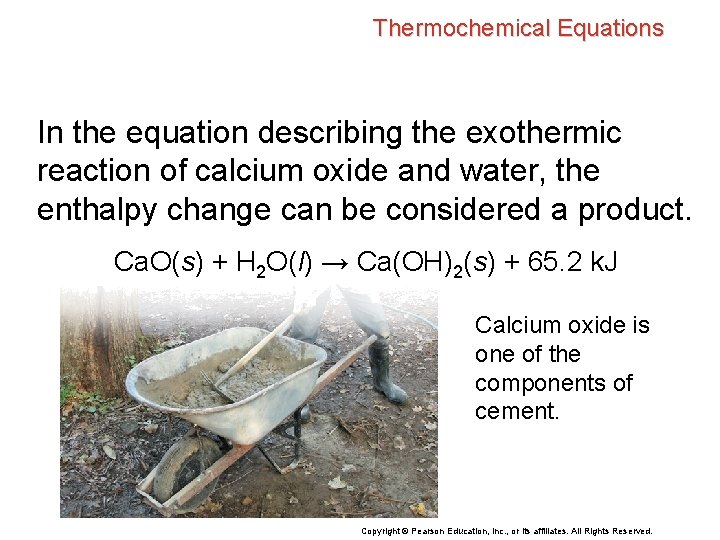
Thermochemical Equations In the equation describing the exothermic reaction of calcium oxide and water, the enthalpy change can be considered a product. Ca. O(s) + H 2 O(l) → Ca(OH)2(s) + 65. 2 k. J Calcium oxide is one of the components of cement. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

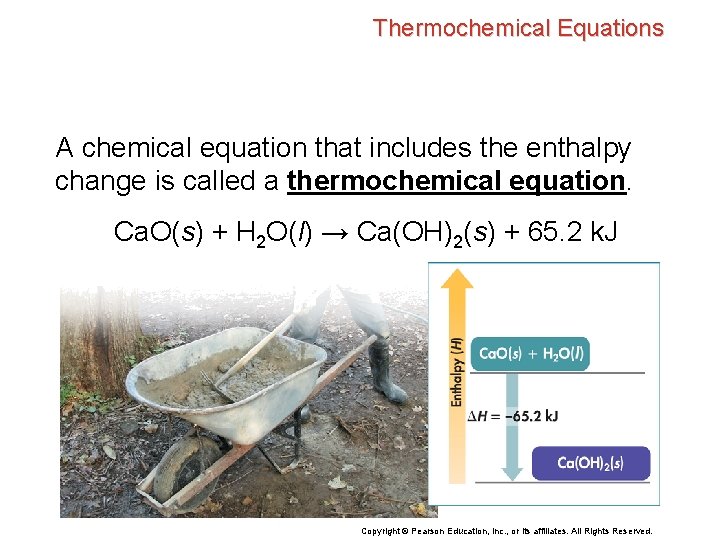
Thermochemical Equations A chemical equation that includes the enthalpy change is called a thermochemical equation. Ca. O(s) + H 2 O(l) → Ca(OH)2(s) + 65. 2 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

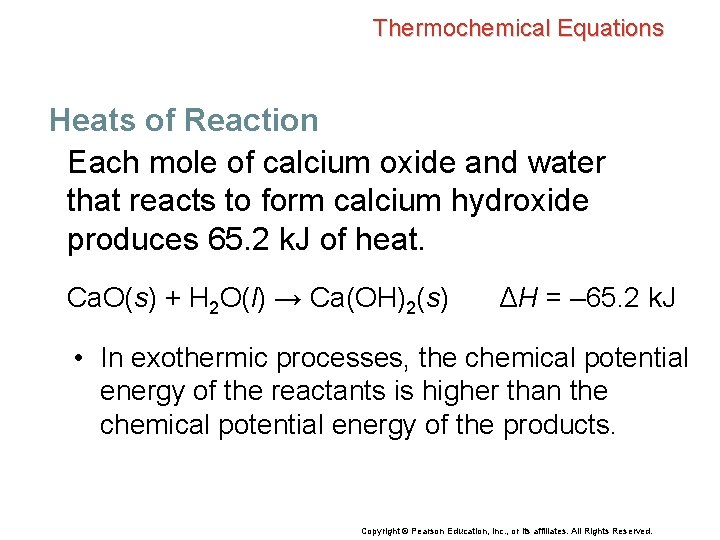
Thermochemical Equations Heats of Reaction The heat of reaction is the enthalpy change for the chemical equation exactly as it is written. • Heats of reaction are reported as ΔH. • The physical state of the reactants and products must also be given. • The standard conditions are that the reaction is carried out at 101. 3 k. Pa (1 atm) and 25°C. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations Heats of Reaction Each mole of calcium oxide and water that reacts to form calcium hydroxide produces 65. 2 k. J of heat. Ca. O(s) + H 2 O(l) → Ca(OH)2(s) ΔH = – 65. 2 k. J • In exothermic processes, the chemical potential energy of the reactants is higher than the chemical potential energy of the products. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

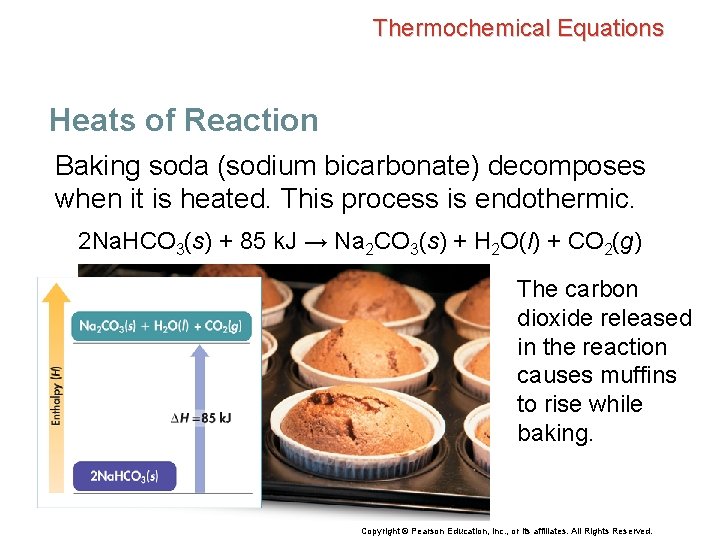
Thermochemical Equations Heats of Reaction Baking soda (sodium bicarbonate) decomposes when it is heated. This process is endothermic. 2 Na. HCO 3(s) + 85 k. J → Na 2 CO 3(s) + H 2 O(l) + CO 2(g) The carbon dioxide released in the reaction causes muffins to rise while baking. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

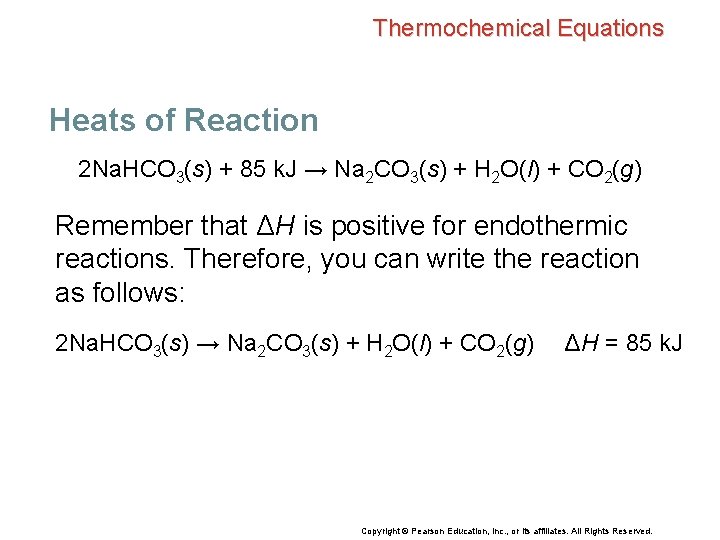
Thermochemical Equations Heats of Reaction 2 Na. HCO 3(s) + 85 k. J → Na 2 CO 3(s) + H 2 O(l) + CO 2(g) Remember that ΔH is positive for endothermic reactions. Therefore, you can write the reaction as follows: 2 Na. HCO 3(s) → Na 2 CO 3(s) + H 2 O(l) + CO 2(g) ΔH = 85 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations Heats of Reaction The amount of heat released or absorbed during a reaction depends on the number of moles of the reactant involved. • The decomposition of 2 mol of sodium bicarbonate requires 85 k. J of heat. • Therefore, the decomposition of 4 mol of the same substance would require twice as much heat, or 170 k. J. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

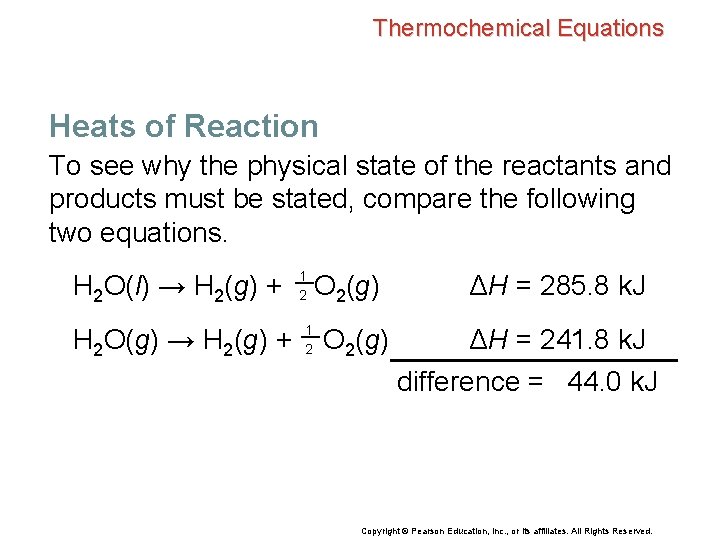
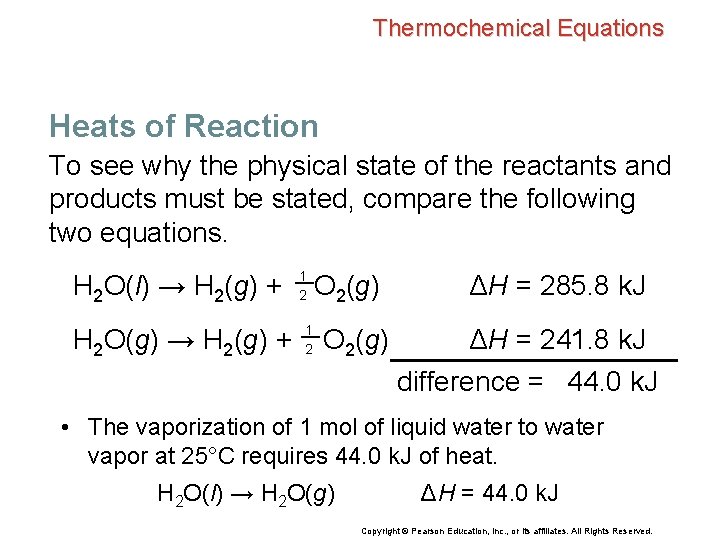
Thermochemical Equations Heats of Reaction To see why the physical state of the reactants and products must be stated, compare the following two equations. H 2 O(l) → H 2(g) + H 2 O(g) → H 2(g) + 1 2 O 2(g) ΔH = 285. 8 k. J ΔH = 241. 8 k. J difference = 44. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations Heats of Reaction To see why the physical state of the reactants and products must be stated, compare the following two equations. H 2 O(l) → H 2(g) + H 2 O(g) → H 2(g) + 1 2 O 2(g) ΔH = 285. 8 k. J ΔH = 241. 8 k. J difference = 44. 0 k. J • The vaporization of 1 mol of liquid water to water vapor at 25°C requires 44. 0 k. J of heat. H 2 O(l) → H 2 O(g) ΔH = 44. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

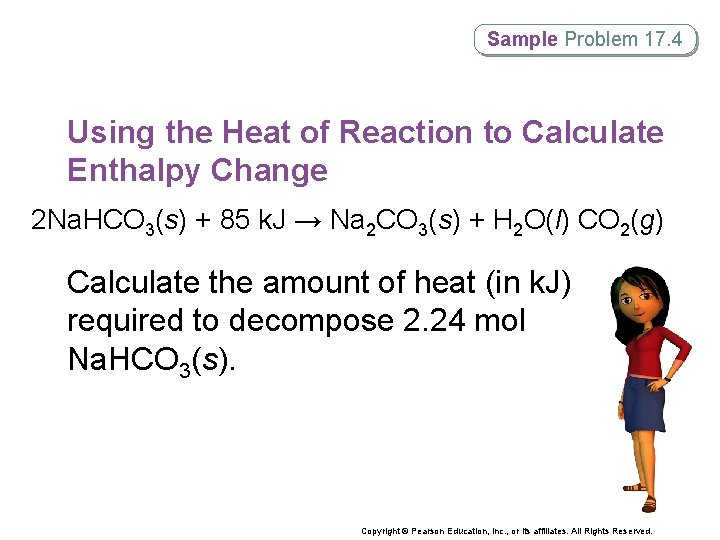
Sample Problem 17. 4 Using the Heat of Reaction to Calculate Enthalpy Change 2 Na. HCO 3(s) + 85 k. J → Na 2 CO 3(s) + H 2 O(l) CO 2(g) Calculate the amount of heat (in k. J) required to decompose 2. 24 mol Na. HCO 3(s). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

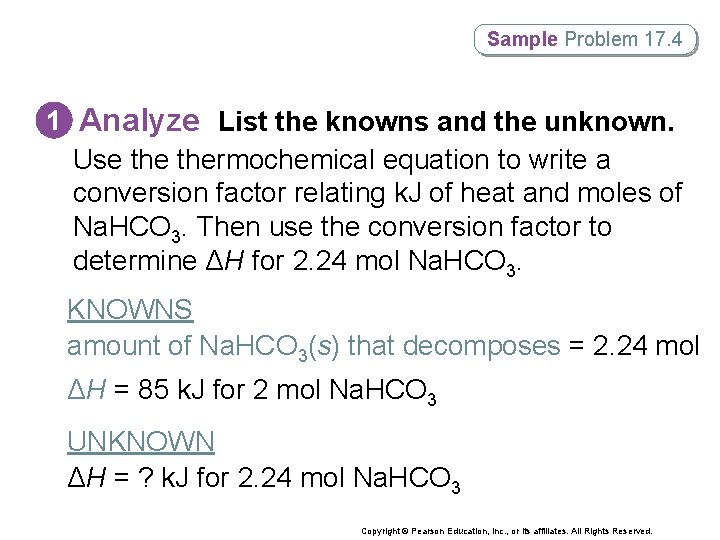
Sample Problem 17. 4 1 Analyze List the knowns and the unknown. Use thermochemical equation to write a conversion factor relating k. J of heat and moles of Na. HCO 3. Then use the conversion factor to determine ΔH for 2. 24 mol Na. HCO 3. KNOWNS amount of Na. HCO 3(s) that decomposes = 2. 24 mol ΔH = 85 k. J for 2 mol Na. HCO 3 UNKNOWN ΔH = ? k. J for 2. 24 mol Na. HCO 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

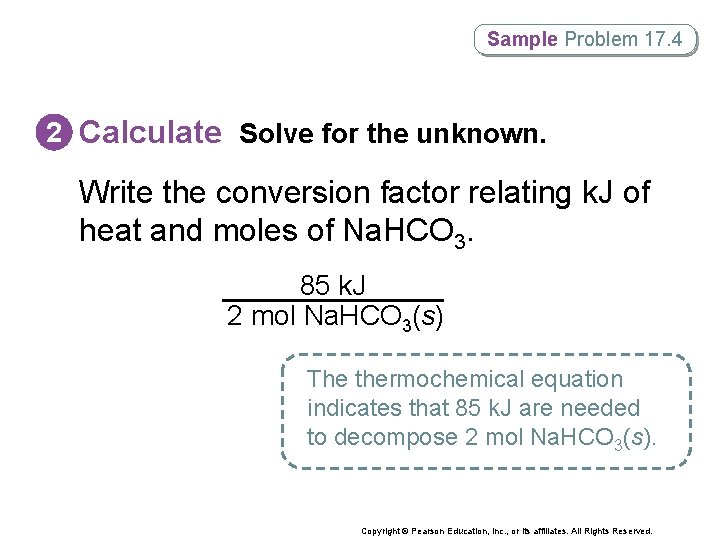
Sample Problem 17. 4 2 Calculate Solve for the unknown. Write the conversion factor relating k. J of heat and moles of Na. HCO 3. 85 k. J 2 mol Na. HCO 3(s) The thermochemical equation indicates that 85 k. J are needed to decompose 2 mol Na. HCO 3(s). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

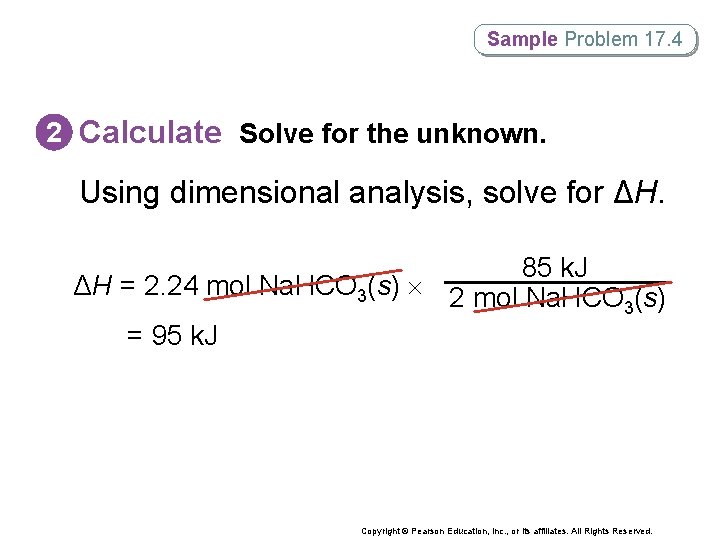
Sample Problem 17. 4 2 Calculate Solve for the unknown. Using dimensional analysis, solve for ΔH. 85 k. J ΔH = 2. 24 mol Na. HCO 3(s) 2 mol Na. HCO (s) 3 = 95 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 4 3 Evaluate Does the result make sense? • The 85 k. J in thermochemical equation refers to the decomposition of 2 mol Na. HCO 3(s). • Therefore, the decomposition of 2. 24 mol should absorb more heat than 85 k. J. • The answer of 95 k. J is consistent with this estimate. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Thermochemical Equations Heats of Combustion The heat of combustion is the heat of reaction for the complete burning of one mole of a substance. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

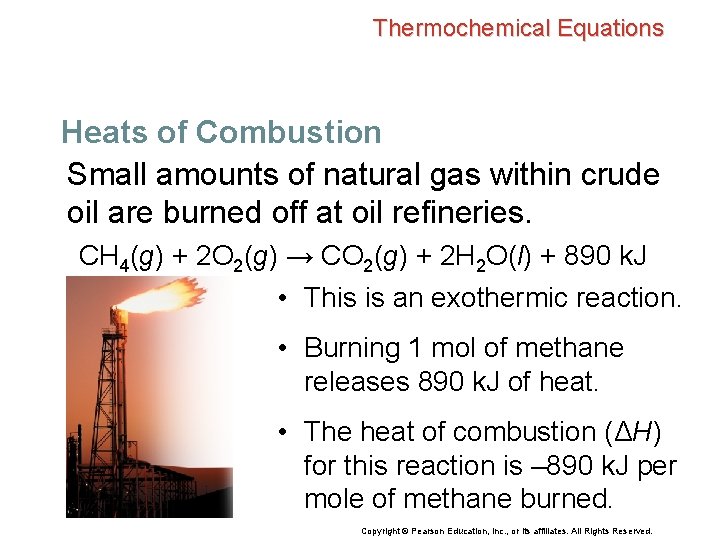
Thermochemical Equations Heats of Combustion Small amounts of natural gas within crude oil are burned off at oil refineries. CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) + 890 k. J • This is an exothermic reaction. • Burning 1 mol of methane releases 890 k. J of heat. • The heat of combustion (ΔH) for this reaction is – 890 k. J per mole of methane burned. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

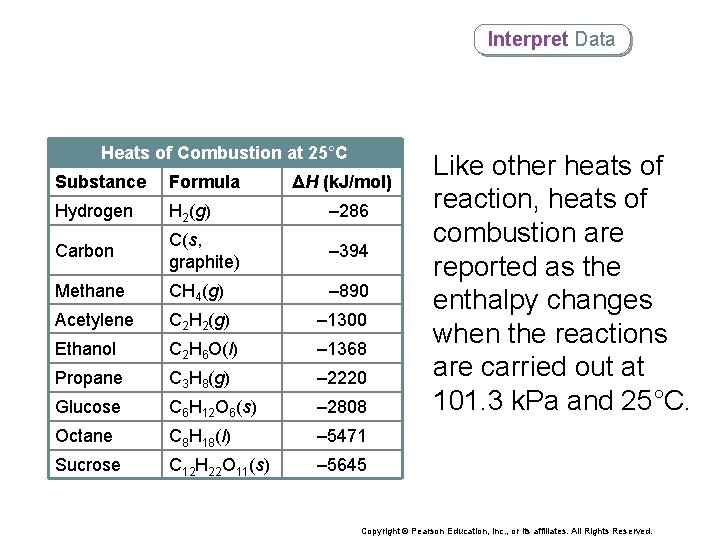
Interpret Data Heats of Combustion at 25°C Substance Formula ΔH (k. J/mol) Hydrogen H 2(g) – 286 Carbon C(s, graphite) – 394 Methane CH 4(g) – 890 Acetylene C 2 H 2(g) – 1300 Ethanol C 2 H 6 O(l) – 1368 Propane C 3 H 8(g) – 2220 Glucose C 6 H 12 O 6(s) – 2808 Octane C 8 H 18(l) – 5471 Sucrose C 12 H 22 O 11(s) – 5645 Like other heats of reaction, heats of combustion are reported as the enthalpy changes when the reactions are carried out at 101. 3 k. Pa and 25°C. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

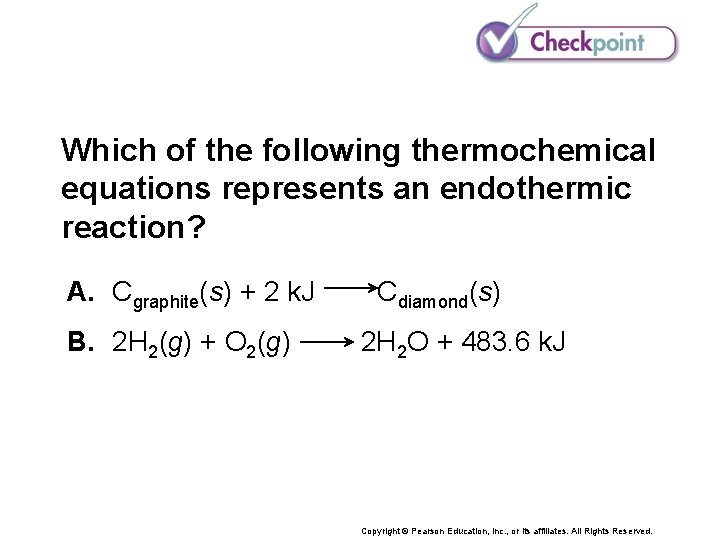
Which of the following thermochemical equations represents an endothermic reaction? A. Cgraphite(s) + 2 k. J B. 2 H 2(g) + O 2(g) Cdiamond(s) 2 H 2 O + 483. 6 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

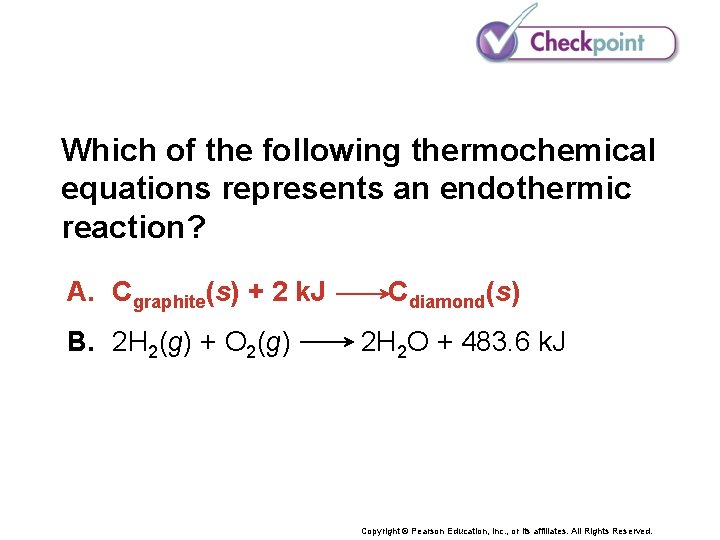
Which of the following thermochemical equations represents an endothermic reaction? A. Cgraphite(s) + 2 k. J B. 2 H 2(g) + O 2(g) Cdiamond(s) 2 H 2 O + 483. 6 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

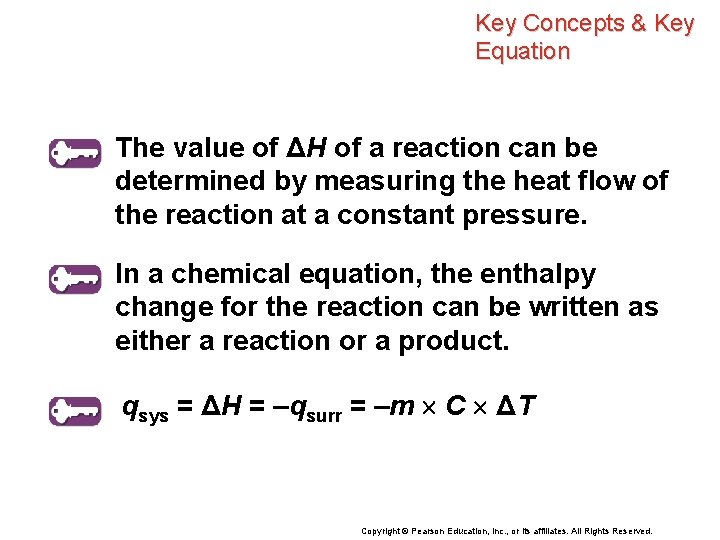
Key Concepts & Key Equation The value of ΔH of a reaction can be determined by measuring the heat flow of the reaction at a constant pressure. In a chemical equation, the enthalpy change for the reaction can be written as either a reaction or a product. qsys = ΔH = –qsurr = –m C ΔT Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • calorimetry: the precise measurement of heat flow out of a system for chemical and physical processes • calorimeter: an insulated device used to measure the absorption or release of heat in chemical or physical processes • enthalpy (H): the heat content of a system at constant pressure Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • thermochemical equation: a chemical equation that includes the enthalpy change • heat of reaction: the enthalpy change for a chemical equation exactly as it is written • heat of combustion: the heat of reaction for the complete burning of one mole of a substance Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

BIG IDEA Matter and Energy The heat of reaction or process can be determined experimentally through calorimetry. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Chapter 17 Thermochemistry 17. 1 The Flow of Energy 17. 2 Measuring and Expressing Enthalpy Changes 17. 3 Heat in Changes of State 17. 4 Calculating Heats of Reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Why does sweating help cool you off? When your body heats up, you start to sweat. The evaporation of sweat is your body’s way of cooling itself to a normal temperature. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Fusion and Solidification What is the relationship between molar heat of fusion and molar heat of solidification? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Fusion and Solidification All solids absorb heat as they melt to become liquids. • The gain of heat causes a change of state instead of a change in temperature. • The temperature of the substance undergoing the change remains constant. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

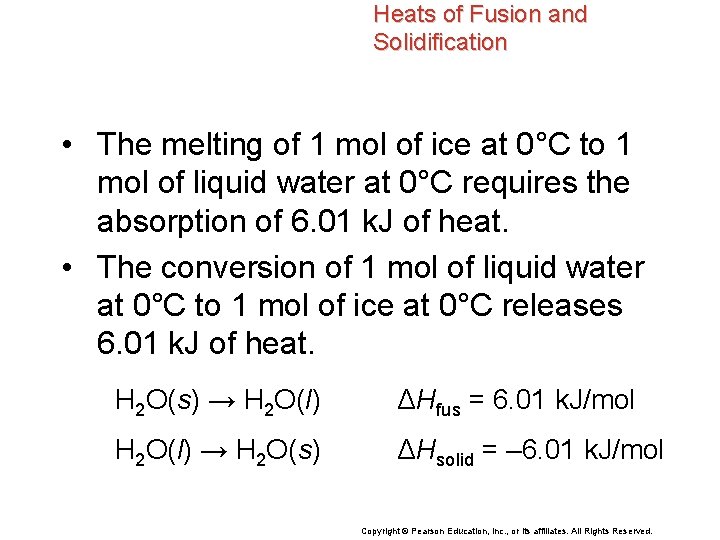
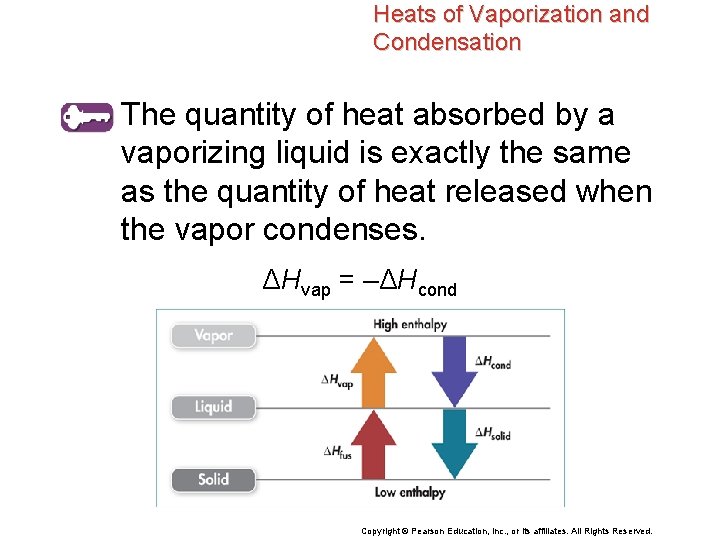
Heats of Fusion and Solidification • The heat absorbed by one mole of a solid substance as it melts to a liquid at constant temperature is the molar heat of fusion (ΔHfus). • The molar heat of solidification (ΔHsolid) is the heat lost when one mole of a liquid substance solidifies at a constant temperature. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Fusion and Solidification The quantity of heat absorbed by a melting solid is exactly the same as the quantity of heat released when the liquid solidifies. ΔHfus = –ΔHsolid Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Fusion and Solidification • The melting of 1 mol of ice at 0°C to 1 mol of liquid water at 0°C requires the absorption of 6. 01 k. J of heat. • The conversion of 1 mol of liquid water at 0°C to 1 mol of ice at 0°C releases 6. 01 k. J of heat. H 2 O(s) → H 2 O(l) ΔHfus = 6. 01 k. J/mol H 2 O(l) → H 2 O(s) ΔHsolid = – 6. 01 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

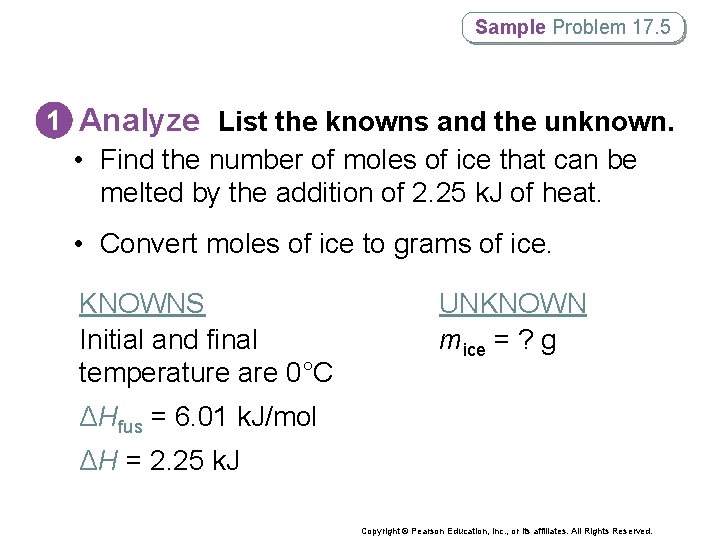
Sample Problem 17. 5 Using the Heat of Fusion in Phase. Change Calculations How many grams of ice at 0°C will melt if 2. 25 k. J of heat are added? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 5 1 Analyze List the knowns and the unknown. • Find the number of moles of ice that can be melted by the addition of 2. 25 k. J of heat. • Convert moles of ice to grams of ice. KNOWNS Initial and final temperature are 0°C UNKNOWN mice = ? g ΔHfus = 6. 01 k. J/mol ΔH = 2. 25 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

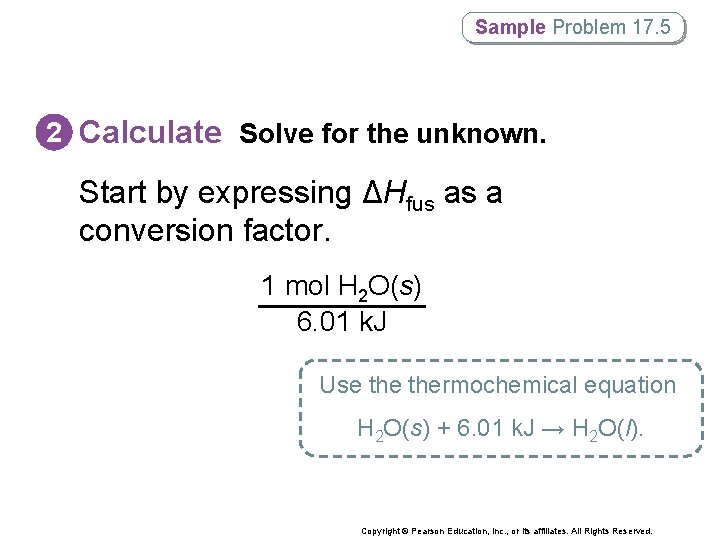
Sample Problem 17. 5 2 Calculate Solve for the unknown. Start by expressing ΔHfus as a conversion factor. 1 mol H 2 O(s) 6. 01 k. J Use thermochemical equation H 2 O(s) + 6. 01 k. J → H 2 O(l). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

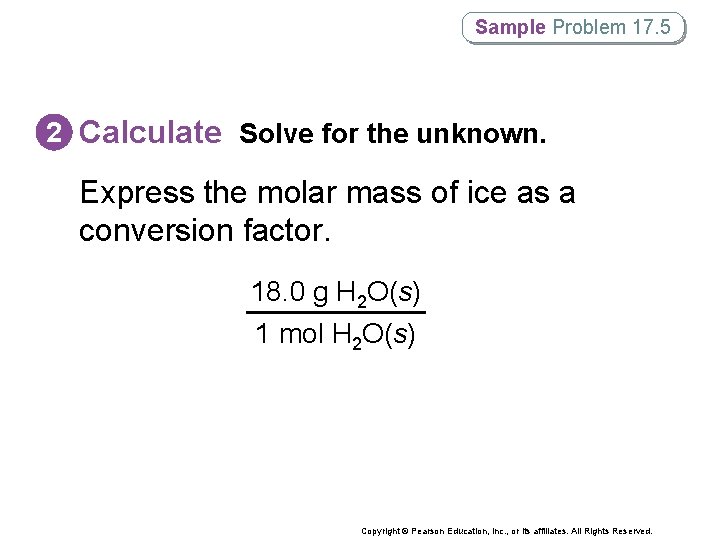
Sample Problem 17. 5 2 Calculate Solve for the unknown. Express the molar mass of ice as a conversion factor. 18. 0 g H 2 O(s) 1 mol H 2 O(s) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

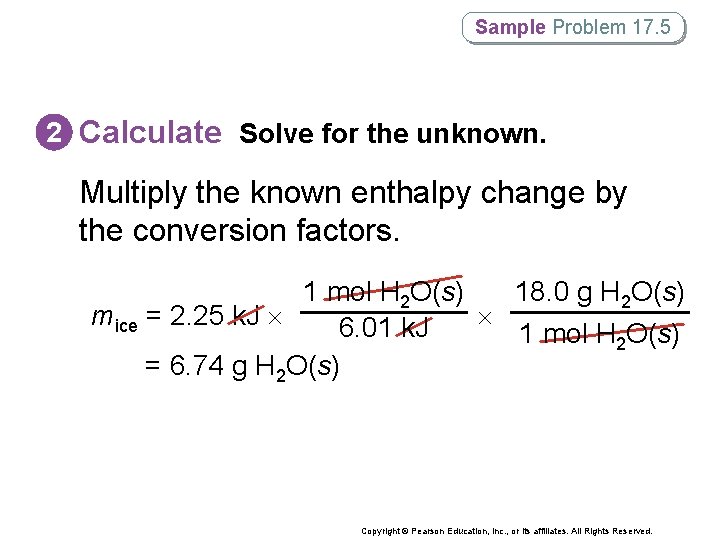
Sample Problem 17. 5 2 Calculate Solve for the unknown. Multiply the known enthalpy change by the conversion factors. 1 mol H 2 O(s) 18. 0 g H 2 O(s) mice = 2. 25 k. J 6. 01 k. J 1 mol H 2 O(s) = 6. 74 g H 2 O(s) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 5 3 Evaluate Does the result make sense? • To melt 1 mol of ice, 6. 01 k. J of energy is required. • Only about one-third of this amount of heat (roughly 2 k. J) is available. • So, only about one-third mol of ice, or 18. 0 g/3 = 6 g, should melt. • This estimate is close to the calculated answer. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

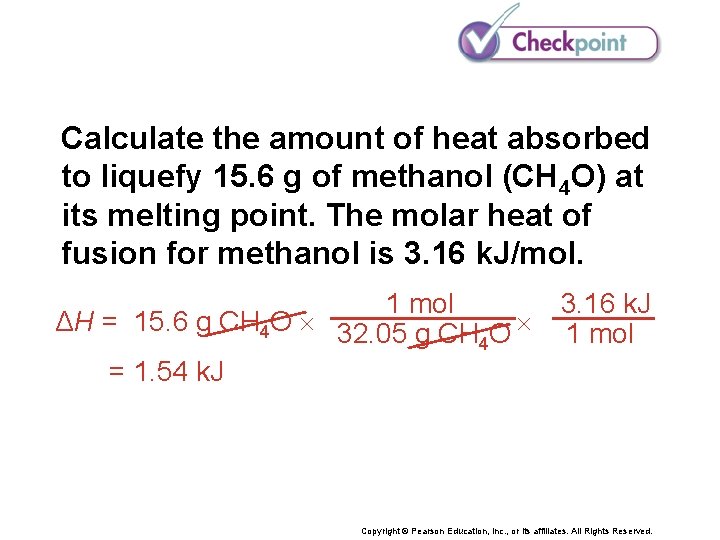
Calculate the amount of heat absorbed to liquefy 15. 0 g of methanol (CH 4 O) at its melting point. The molar heat of fusion for methanol is 3. 16 k. J/mol. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calculate the amount of heat absorbed to liquefy 15. 6 g of methanol (CH 4 O) at its melting point. The molar heat of fusion for methanol is 3. 16 k. J/mol. 1 mol ΔH = 15. 6 g CH 4 O 32. 05 g CH O 4 = 1. 54 k. J 3. 16 k. J 1 mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Vaporization and Condensation What is the relationship between molar heat of vaporization and molar heat of condensation? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Vaporization and Condensation A liquid that absorbs heat at its boiling point becomes a vapor. • The amount of heat required to vaporize one mole of a given liquid at a constant temperature is called its molar heat of vaporization (ΔHvap). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

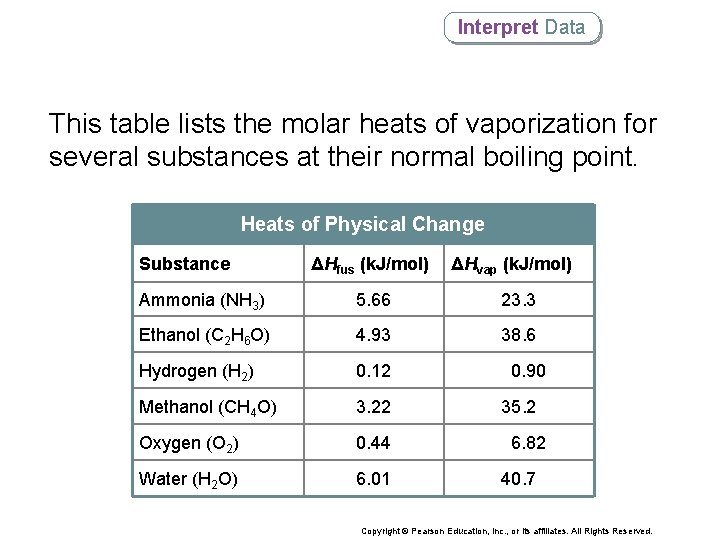
Interpret Data This table lists the molar heats of vaporization for several substances at their normal boiling point. Heats of Physical Change Substance ΔHfus (k. J/mol) ΔHvap (k. J/mol) Ammonia (NH 3) 5. 66 23. 3 Ethanol (C 2 H 6 O) 4. 93 38. 6 Hydrogen (H 2) 0. 12 Methanol (CH 4 O) 3. 22 Oxygen (O 2) 0. 44 Water (H 2 O) 6. 01 0. 90 35. 2 6. 82 40. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Vaporization and Condensation is the exact opposite of vaporization. • When a vapor condenses, heat is released. • The molar heat of condensation (ΔHcond) is the amount of heat released when one mole of vapor condenses at its normal boiling point. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heats of Vaporization and Condensation The quantity of heat absorbed by a vaporizing liquid is exactly the same as the quantity of heat released when the vapor condenses. ΔHvap = –ΔHcond Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Explain why the evaporation of sweat off your body helps cool you off. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Explain why the evaporation of sweat off your body helps cool you off. Energy is required to vaporize (or evaporate) a liquid into a gas. When liquid sweat absorbs energy from your skin, the temperature of your skin decreases. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

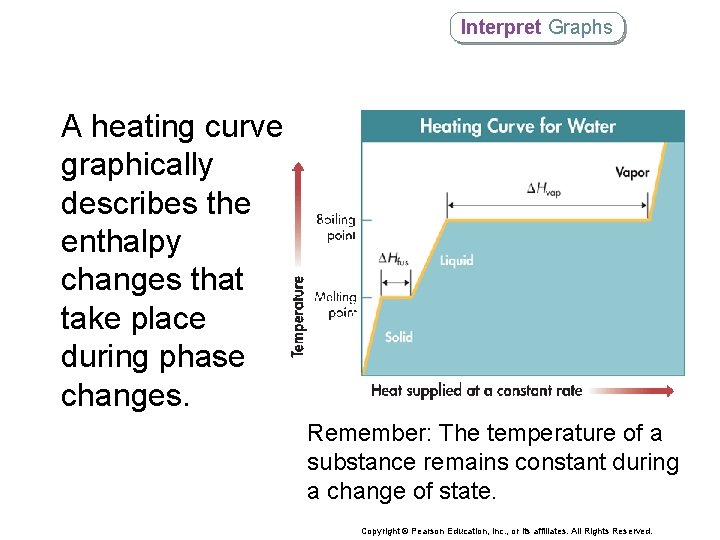
Interpret Graphs A heating curve graphically describes the enthalpy changes that take place during phase changes. Remember: The temperature of a substance remains constant during a change of state. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

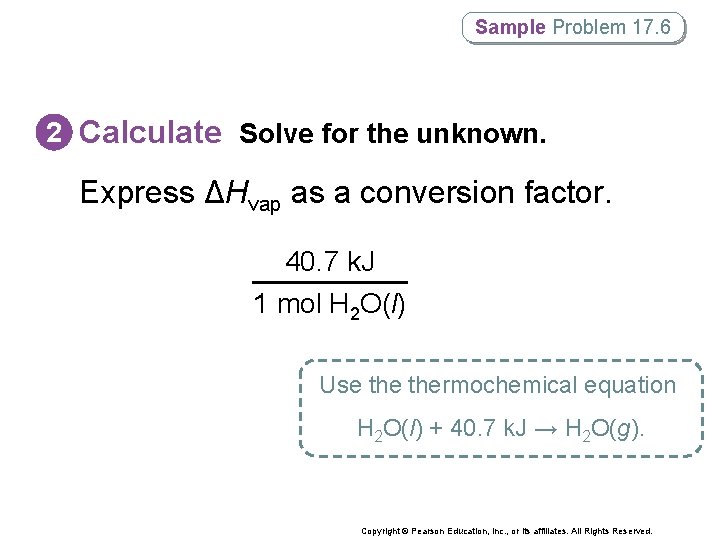
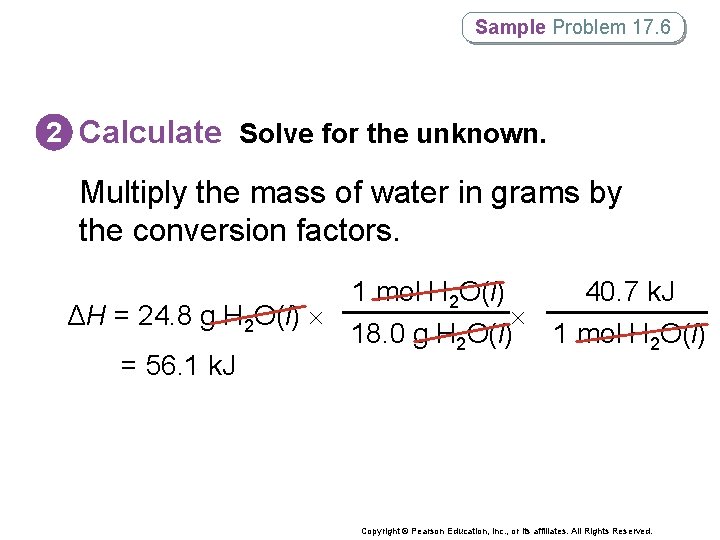
Sample Problem 17. 6 Using the Heat of Vaporization in Phase. Change Calculations How much heat (in k. J) is absorbed when 24. 8 g H 2 O(l) at 100°C and 101. 3 k. Pa is converted to H 2 O(g) at 100°C? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

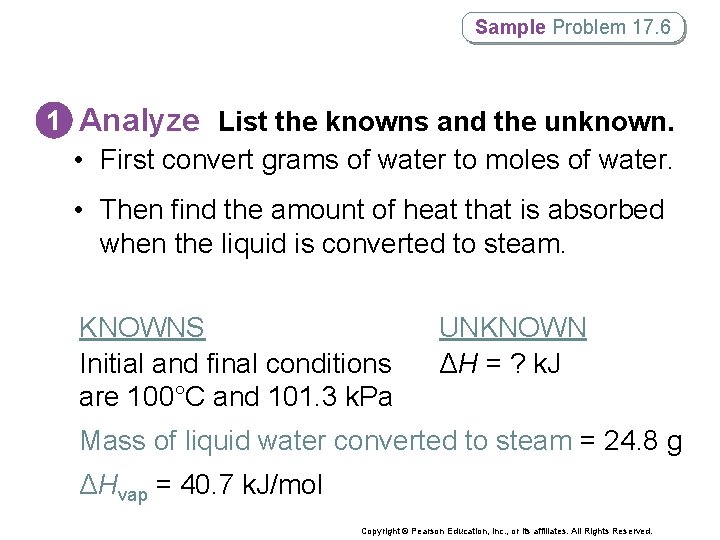
Sample Problem 17. 6 1 Analyze List the knowns and the unknown. • First convert grams of water to moles of water. • Then find the amount of heat that is absorbed when the liquid is converted to steam. KNOWNS Initial and final conditions are 100°C and 101. 3 k. Pa UNKNOWN ΔH = ? k. J Mass of liquid water converted to steam = 24. 8 g ΔHvap = 40. 7 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 6 2 Calculate Solve for the unknown. Start by expressing the molar mass of water as a conversion factor. 1 mol H 2 O(l) 18. 0 g H 2 O(l) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 6 2 Calculate Solve for the unknown. Express ΔHvap as a conversion factor. 40. 7 k. J 1 mol H 2 O(l) Use thermochemical equation H 2 O(l) + 40. 7 k. J → H 2 O(g). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 6 2 Calculate Solve for the unknown. Multiply the mass of water in grams by the conversion factors. ΔH = 24. 8 g H 2 O(l) = 56. 1 k. J 1 mol H 2 O(l) 40. 7 k. J 18. 0 g H 2 O(l) 1 mol H 2 O(l) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 6 3 Evaluate Does the result make sense? • Knowing that the molar mass of water is 18. 0 g/mol, 24. 8 g H 2 O(l) can be estimated to be somewhat less than 1. 5 mol H 2 O. • The calculated enthalpy change should be a little less than 1. 5 mol 40 k. J/mol = 60 k. J, and it is. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The molar heat of condensation of a substance is the same, in magnitude, as which of the following? A. molar heat of fusion B. molar heat of vaporization C. molar heat of solidification D. molar heat of formation Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

The molar heat of condensation of a substance is the same, in magnitude, as which of the following? A. molar heat of fusion B. molar heat of vaporization C. molar heat of solidification D. molar heat of formation Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat of Solution What thermochemical changes can occur when a solution forms? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat of Solution During the formation of a solution, heat is either released or absorbed. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat of Solution During the formation of a solution, heat is either released or absorbed. • The enthalpy change caused by the dissolution of one mole of substance is the molar heat of solution (ΔHsoln). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat of Solution A practical application of an exothermic dissolution process is a hot pack. • In a hot pack, calcium chloride, Ca. Cl 2(s), mixes with water, producing heat. Ca. Cl 2(s) → Ca 2+(aq) + 2 Cl–(aq) ΔHsoln = – 82. 8 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat of Solution The dissolution of ammonium nitrate, NH 4 NO 3(s), is an example of an endothermic process. • The cold pack shown here contains solid ammonium nitrate crystals and water. • Once the solute dissolves, the pack becomes cold. • The solution process absorbs energy from the surroundings. NH 4 NO 3(s) → NH 4+(aq) + NO 3–(aq) ΔHsoln = 25. 7 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

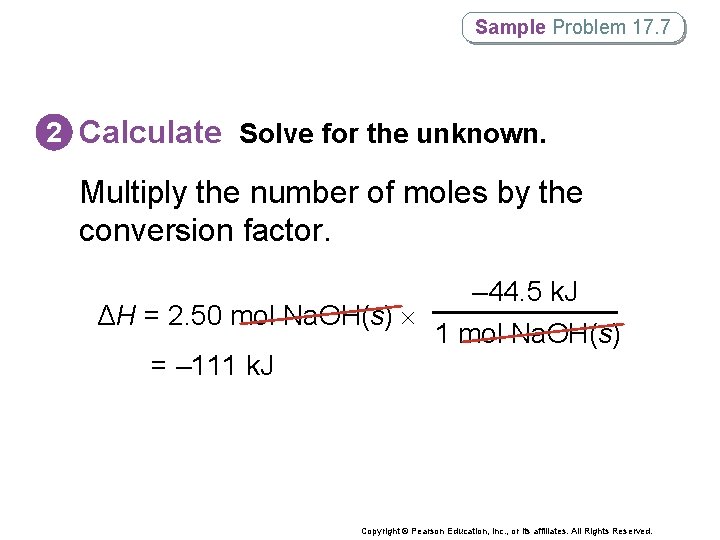
Sample Problem 17. 7 Calculating the Enthalpy Change in Solution Formation How much heat (in k. J) is released when 2. 50 mol Na. OH(s) is dissolved in water? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 7 1 Analyze List the knowns and the unknown. Use the heat of solution for the dissolution of Na. OH(s) in water to solve for the amount of heat released (ΔH). KNOWNS ΔHsoln = – 44. 5 k. J/mol amount of Na. OH(s) dissolved = 2. 50 mol UNKNOWN ΔH = ? k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

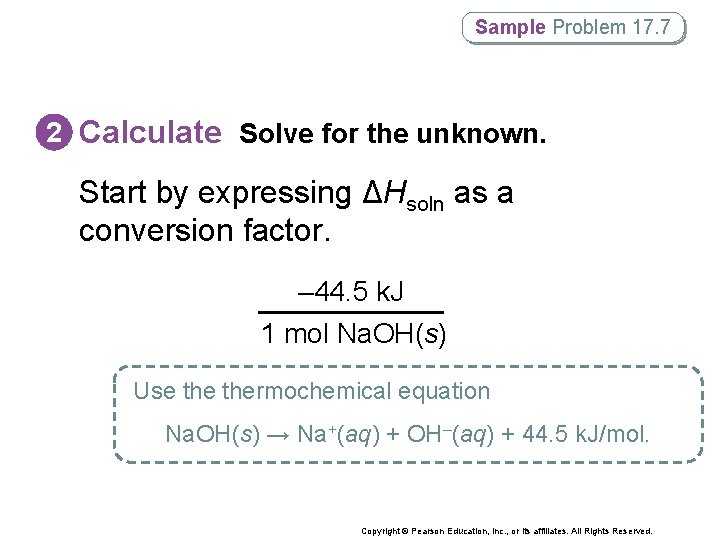
Sample Problem 17. 7 2 Calculate Solve for the unknown. Start by expressing ΔHsoln as a conversion factor. – 44. 5 k. J 1 mol Na. OH(s) Use thermochemical equation Na. OH(s) → Na+(aq) + OH–(aq) + 44. 5 k. J/mol. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 7 2 Calculate Solve for the unknown. Multiply the number of moles by the conversion factor. ΔH = 2. 50 mol Na. OH(s) – 44. 5 k. J 1 mol Na. OH(s) = – 111 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 7 3 Evaluate Does the result make sense? • ΔH is 2. 5 times greater than ΔHsoln, as it should be. • Also, ΔH should be negative, as the dissolution of Na. OH(s) in water is exothermic. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

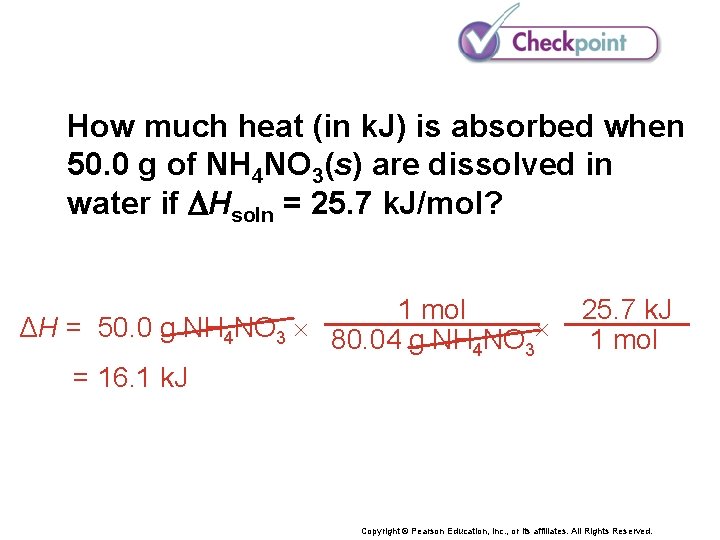
How much heat (in k. J) is absorbed when 50. 0 g of NH 4 NO 3(s) are dissolved in water if Hsoln = 25. 7 k. J/mol? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

How much heat (in k. J) is absorbed when 50. 0 g of NH 4 NO 3(s) are dissolved in water if Hsoln = 25. 7 k. J/mol? 1 mol ΔH = 50. 0 g NH 4 NO 3 80. 04 g NH NO 4 3 = 16. 1 k. J 25. 7 k. J 1 mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts The quantity of heat absorbed by a melting solid is exactly the same as the quantity of heat released when the liquid solidifies; that is, ΔHfus = –ΔHsolid. The quantity of heat absorbed by a vaporizing liquid is exactly the same as the quantity of heat released when the vapor condenses; that is, ΔHvap = –ΔHcond. During the formation of a solution, heat is either released or absorbed. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • molar heat of fusion (ΔHfus): the amount of heat absorbed by one mole of a solid substance as it melts to a liquid at a constant temperature • molar heat of solidification (ΔHsolid): the amount of heat lost by one mole of a liquid as it solidifies at a constant temperature • molar heat of vaporization (ΔHvap): the amount of heat absorbed by one mole of a liquid as it vaporizes at a constant temperature Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • molar heat of condensation (ΔHcond): the amount of heat released by one mole of a vapor as it condenses to a liquid at a constant temperature • molar heat of solution (ΔHsoln): the enthalpy change caused by the dissolution of one mole of a substance Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Chapter 17 Thermochemistry 17. 1 The Flow of Energy 17. 2 Measuring and Expressing Enthalpy Changes 17. 3 Heat in Changes of State 17. 4 Calculating Heats of Reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU How much heat is released when a diamond changes into graphite? Diamonds are gemstones composed of carbon. Over a time period of millions and millions of years, diamond will break down into graphite, which is another form of carbon. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law How can you calculate the heat of reaction when it cannot be directly measured? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

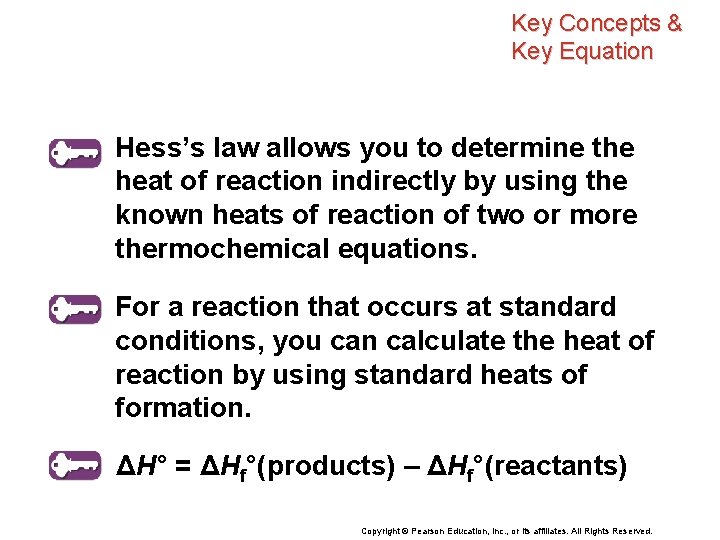
Hess’s Law Hess’s law of heat summation states that if you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law Hess’s law allows you to determine the heat of reaction indirectly by using the known heats of reaction of two or more thermochemical equations. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

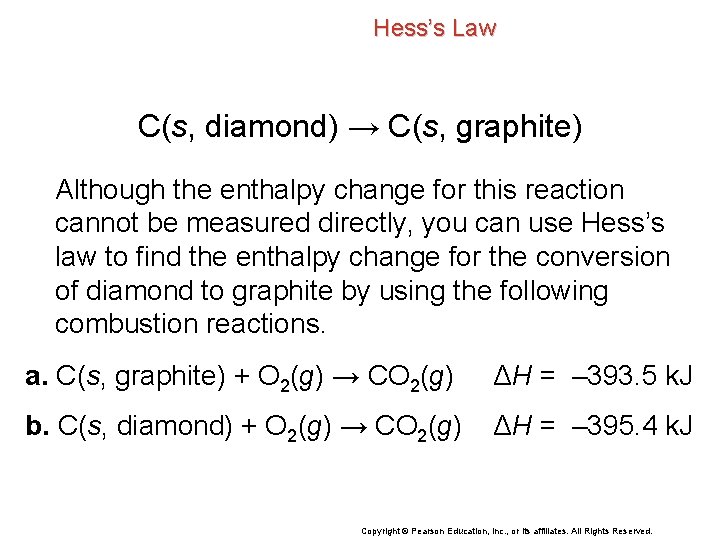
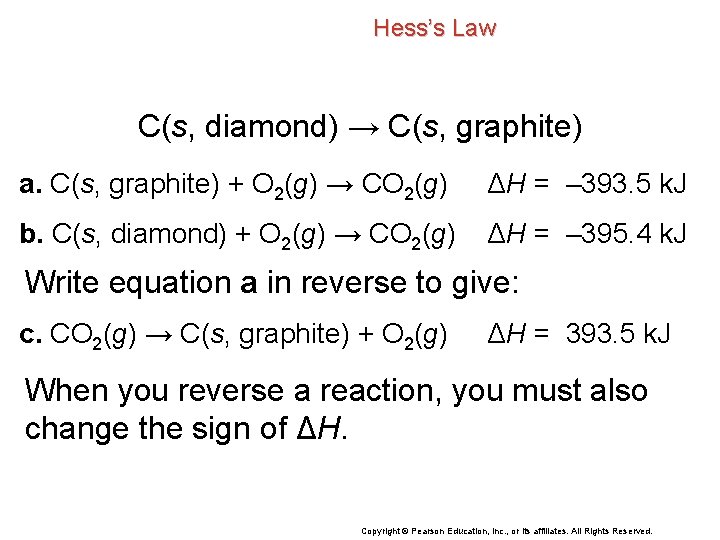
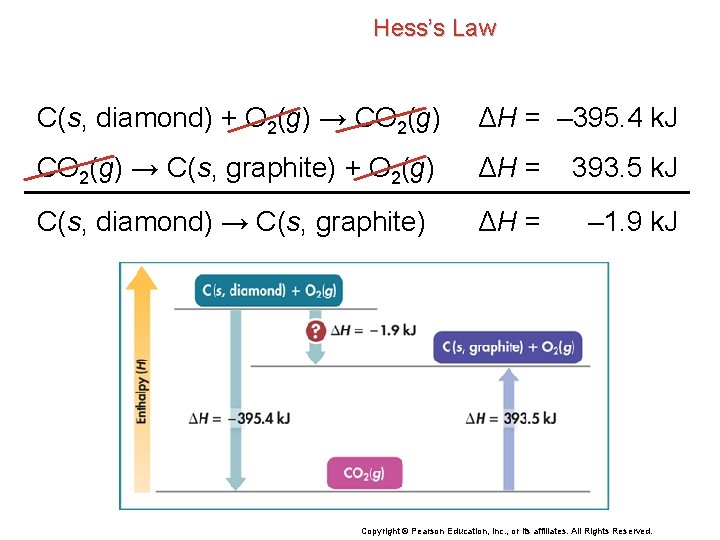
Hess’s Law C(s, diamond) → C(s, graphite) Although the enthalpy change for this reaction cannot be measured directly, you can use Hess’s law to find the enthalpy change for the conversion of diamond to graphite by using the following combustion reactions. a. C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J b. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law C(s, diamond) → C(s, graphite) a. C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J b. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J Write equation a in reverse to give: c. CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J When you reverse a reaction, you must also change the sign of ΔH. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

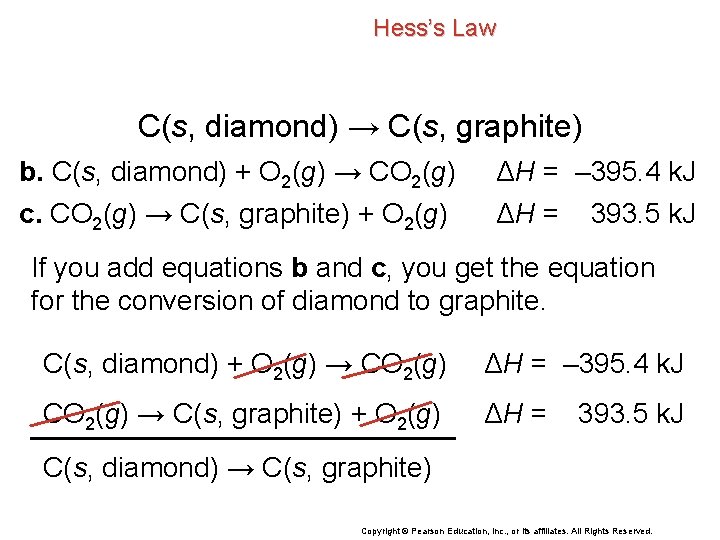
Hess’s Law C(s, diamond) → C(s, graphite) b. C(s, diamond) + O 2(g) → CO 2(g) c. CO 2(g) → C(s, graphite) + O 2(g) ΔH = – 395. 4 k. J ΔH = 393. 5 k. J If you add equations b and c, you get the equation for the conversion of diamond to graphite. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

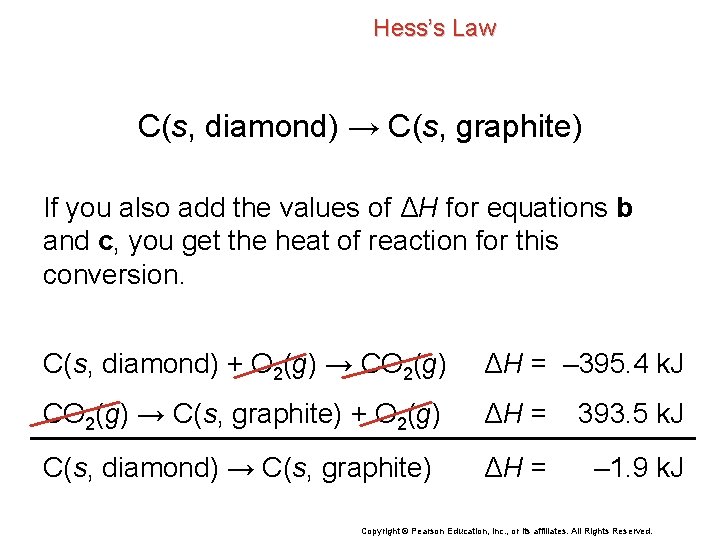
Hess’s Law C(s, diamond) → C(s, graphite) If you also add the values of ΔH for equations b and c, you get the heat of reaction for this conversion. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) ΔH = – 1. 9 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) ΔH = – 1. 9 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU How can you determine ΔH for the conversion of diamond to graphite without performing the reaction? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU How can you determine ΔH for the conversion of diamond to graphite without performing the reaction? You can use Hess’s law by adding thermochemical equations in which the enthalpy changes are known and whose sum will result in an equation for the conversion of diamond to graphite. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law Another case where Hess’s law is useful is when reactions yield products in addition to the product of interest. • Suppose you want to determine the enthalpy change for the formation of carbon monoxide from its elements. C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = ? • Carrying out the reaction in the laboratory as written is virtually impossible. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

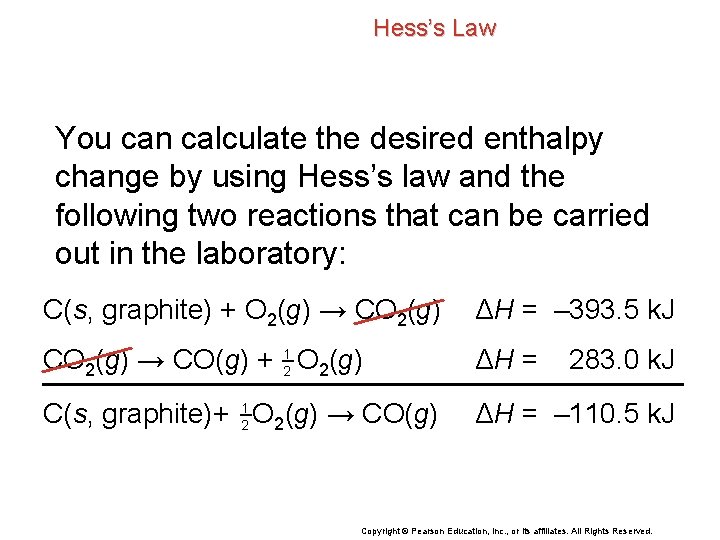
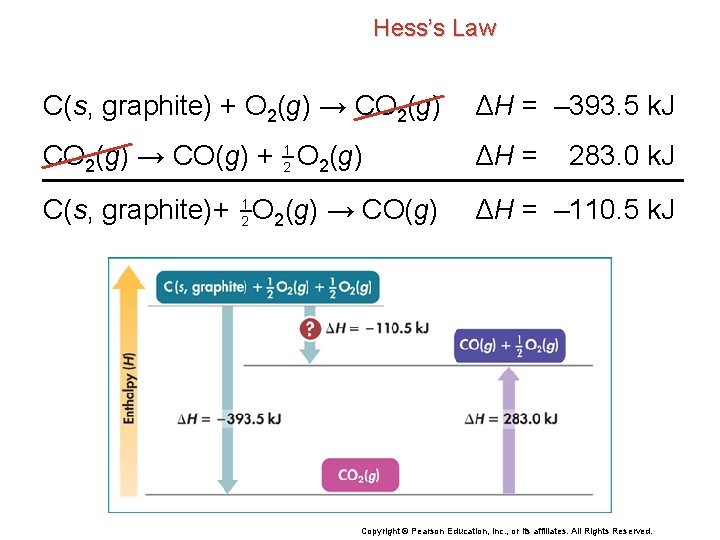
Hess’s Law You can calculate the desired enthalpy change by using Hess’s law and the following two reactions that can be carried out in the laboratory: C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J CO 2(g) → CO(g) + 21 O 2(g) ΔH = C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = – 110. 5 k. J 283. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hess’s Law C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J CO 2(g) → CO(g) + 21 O 2(g) ΔH = C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = – 110. 5 k. J 283. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

According to Hess’s law, it is possible to calculate an unknown heat of reaction by using which of the following? A. heats of fusion for each of the compounds in the reaction B. two other reactions with known heats of reaction C. specific heat capacities for each compound in the reaction D. density for each compound in the reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

According to Hess’s law, it is possible to calculate an unknown heat of reaction by using which of the following? A. heats of fusion for each of the compounds in the reaction B. two other reactions with known heats of reaction C. specific heat capacities for each compound in the reaction D. density for each compound in the reaction Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Standard Heats of Formation How can you calculate the heat of reaction when it cannot be directly measured? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Standard Heats of Formation Enthalpy changes generally depend on the conditions of the process. • Scientists specify a common set of conditions as a reference point. • These conditions, called the standard state, refer to the stable form of a substance at 25°C and 101. 3 k. Pa. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

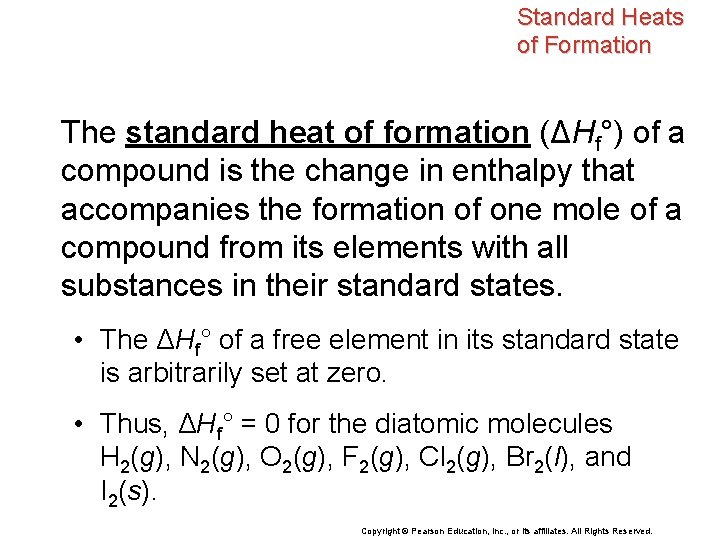
Standard Heats of Formation The standard heat of formation (ΔHf°) of a compound is the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states. • The ΔHf° of a free element in its standard state is arbitrarily set at zero. • Thus, ΔHf° = 0 for the diatomic molecules H 2(g), N 2(g), O 2(g), F 2(g), Cl 2(g), Br 2(l), and I 2(s). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

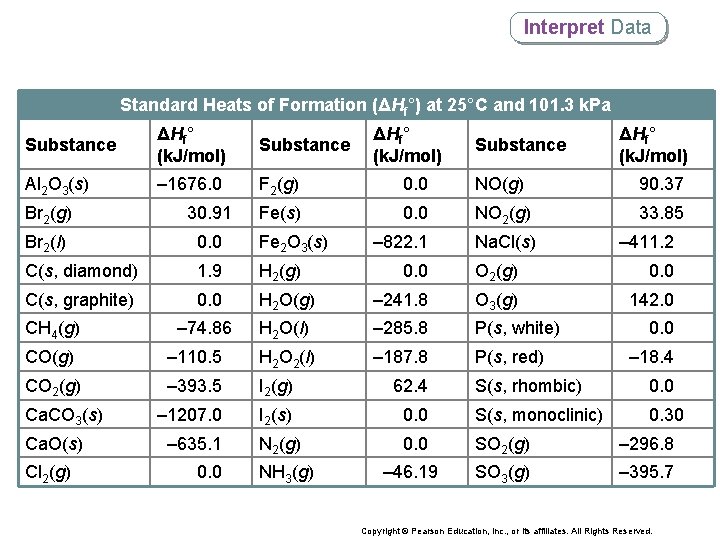
Interpret Data Standard Heats of Formation (ΔHf°) at 25°C and 101. 3 k. Pa Substance ΔHf° (k. J/mol) Substance Al 2 O 3(s) – 1676. 0 F 2(g) 0. 0 NO(g) 90. 37 Fe(s) 0. 0 NO 2(g) 33. 85 – 822. 1 Na. Cl(s) Br 2(g) 30. 91 Br 2(l) 0. 0 Fe 2 O 3(s) C(s, diamond) 1. 9 H 2(g) C(s, graphite) 0. 0 – 74. 86 CH 4(g) ΔHf° (k. J/mol) 0. 0 H 2 O(g) – 241. 8 O 3(g) 142. 0 H 2 O(l) – 285. 8 P(s, white) – 187. 8 P(s, red) H 2 O 2(l) CO 2(g) – 393. 5 I 2(g) 62. 4 – 1207. 0 I 2(s) – 635. 1 N 2(g) Cl 2(g) 0. 0 – 411. 2 O 2(g) – 110. 5 Ca. O(s) ΔHf° (k. J/mol) 0. 0 CO(g) Ca. CO 3(s) Substance NH 3(g) 0. 0 – 18. 4 S(s, rhombic) 0. 0 S(s, monoclinic) 0. 30 0. 0 SO 2(g) – 296. 8 – 46. 19 SO 3(g) – 395. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. • Such an enthalpy change is called the standard heat of reaction (ΔH°). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

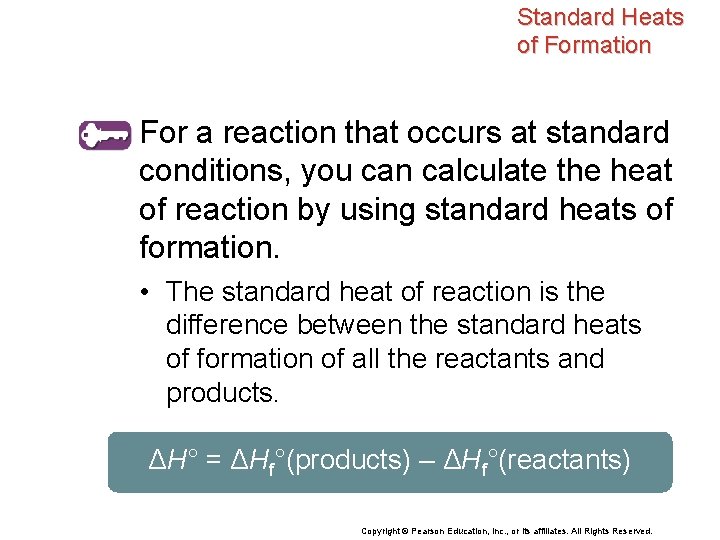
Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. • The standard heat of reaction is the difference between the standard heats of formation of all the reactants and products. ΔH° = ΔHf°(products) – ΔHf°(reactants) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

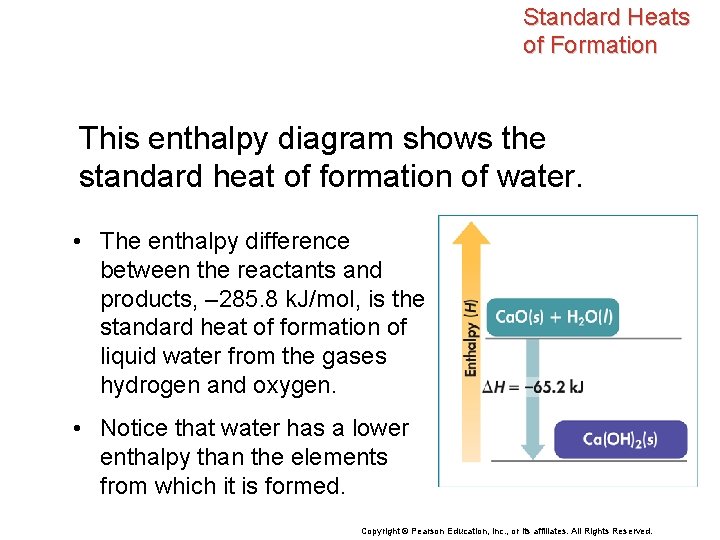
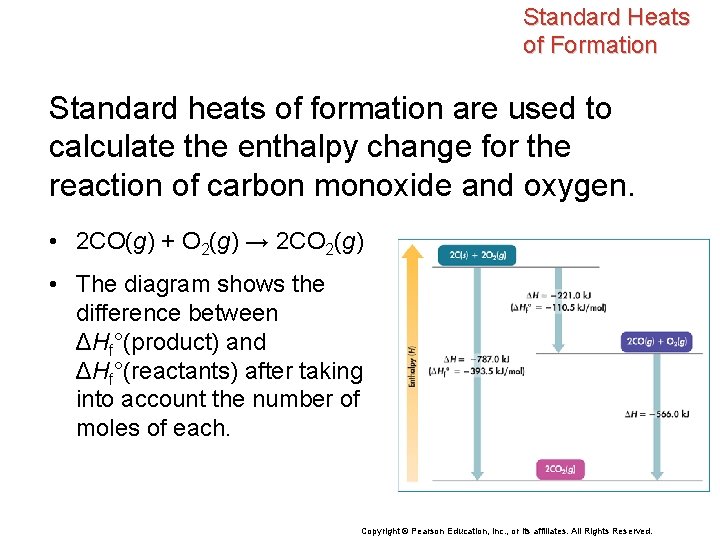
Standard Heats of Formation This enthalpy diagram shows the standard heat of formation of water. • The enthalpy difference between the reactants and products, – 285. 8 k. J/mol, is the standard heat of formation of liquid water from the gases hydrogen and oxygen. • Notice that water has a lower enthalpy than the elements from which it is formed. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

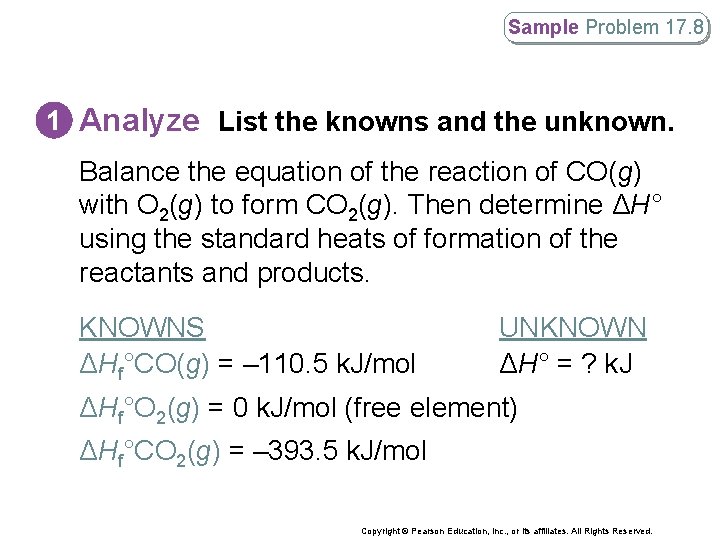
Sample Problem 17. 8 Calculating the Standard Heat of Reaction What is the standard heat of reaction (ΔH°) for the reaction of CO(g) with O 2(g) to form CO 2(g)? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 8 1 Analyze List the knowns and the unknown. Balance the equation of the reaction of CO(g) with O 2(g) to form CO 2(g). Then determine ΔH° using the standard heats of formation of the reactants and products. KNOWNS ΔHf°CO(g) = – 110. 5 k. J/mol UNKNOWN ΔH° = ? k. J ΔHf°O 2(g) = 0 k. J/mol (free element) ΔHf°CO 2(g) = – 393. 5 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

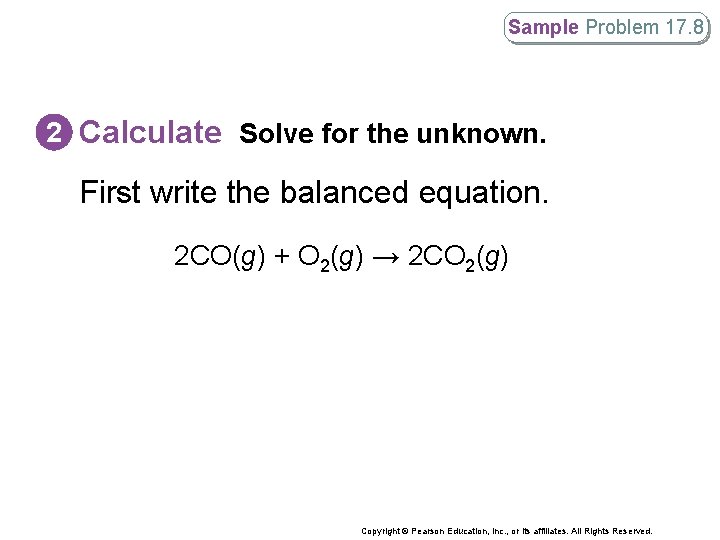
Sample Problem 17. 8 2 Calculate Solve for the unknown. First write the balanced equation. 2 CO(g) + O 2(g) → 2 CO 2(g) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

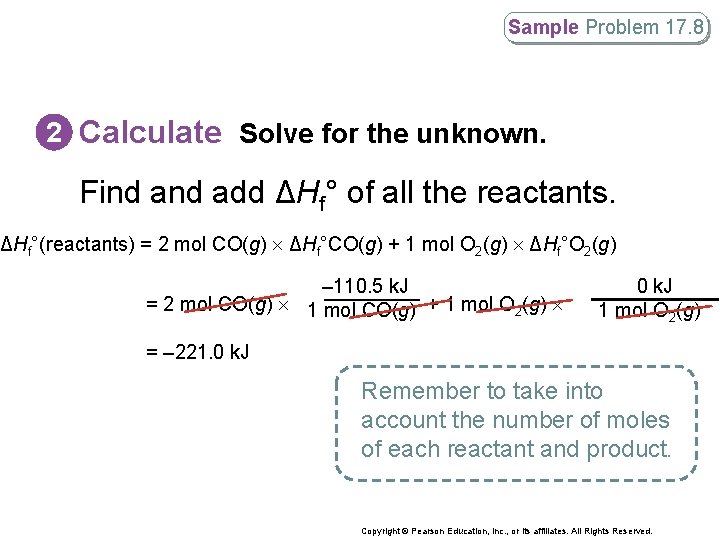
Sample Problem 17. 8 2 Calculate Solve for the unknown. Find add ΔHf° of all the reactants. ΔHf°(reactants) = 2 mol CO(g) ΔHf°CO(g) + 1 mol O 2(g) ΔHf°O 2(g) – 110. 5 k. J = 2 mol CO(g) 1 mol CO(g) + 1 mol O 2(g) 0 k. J 1 mol O 2(g) = – 221. 0 k. J Remember to take into account the number of moles of each reactant and product. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

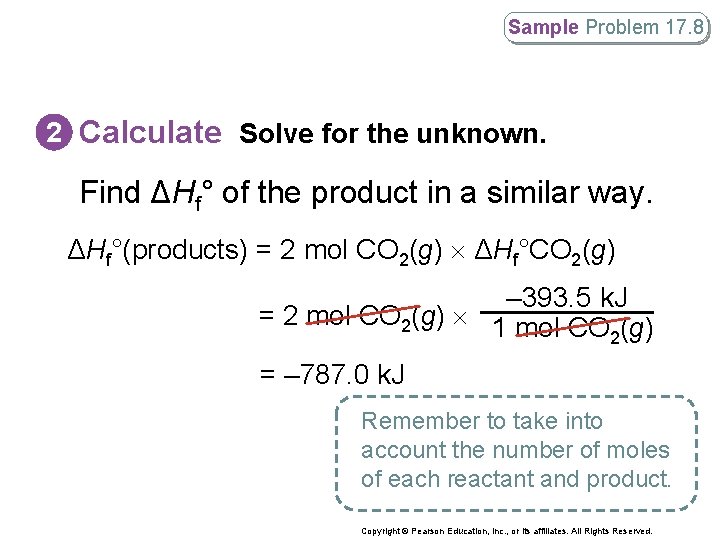
Sample Problem 17. 8 2 Calculate Solve for the unknown. Find ΔHf° of the product in a similar way. ΔHf°(products) = 2 mol CO 2(g) ΔHf°CO 2(g) – 393. 5 k. J = 2 mol CO 2(g) 1 mol CO (g) 2 = – 787. 0 k. J Remember to take into account the number of moles of each reactant and product. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 8 2 Calculate Solve for the unknown. Calculate ΔH° for the reaction. ΔH° = ΔHf°(products) – ΔHf°(reactants) = (– 787. 0 k. J) – (– 221. 0 k. J) = – 566. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 17. 8 3 Evaluate Does the result make sense? • The ΔH° is negative, so the reaction is exothermic. • This outcome makes sense because combustion reactions always release heat. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Standard Heats of Formation Standard heats of formation are used to calculate the enthalpy change for the reaction of carbon monoxide and oxygen. • 2 CO(g) + O 2(g) → 2 CO 2(g) • The diagram shows the difference between ΔHf°(product) and ΔHf°(reactants) after taking into account the number of moles of each. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

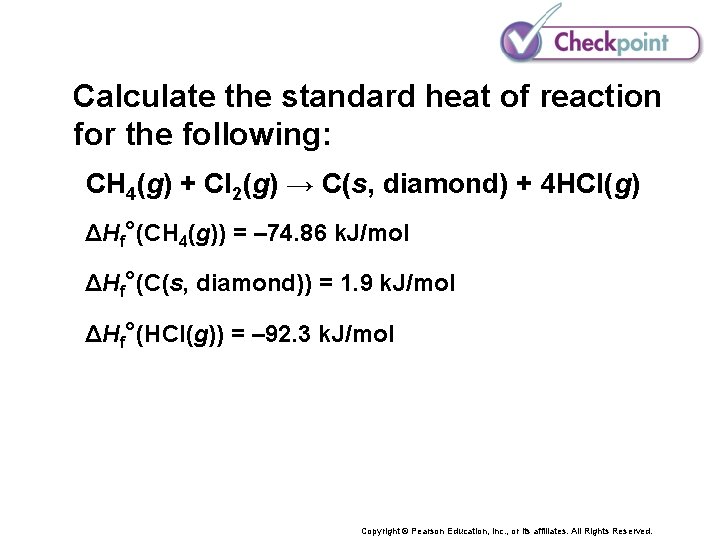
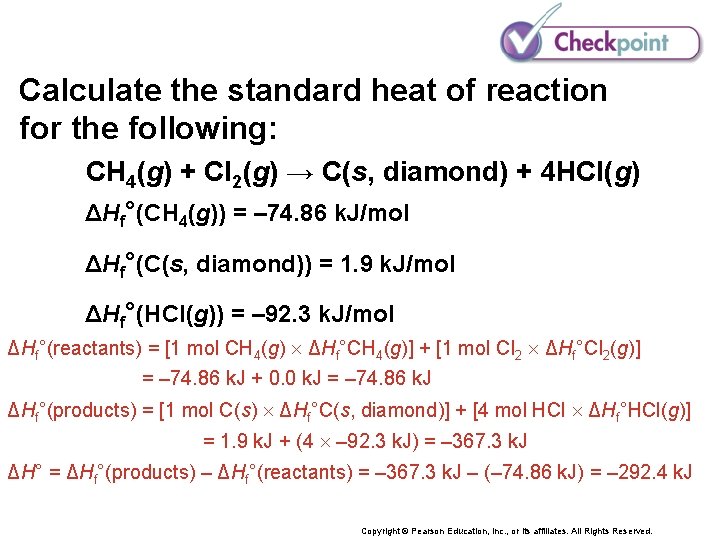
Calculate the standard heat of reaction for the following: CH 4(g) + Cl 2(g) → C(s, diamond) + 4 HCl(g) ΔHf°(CH 4(g)) = – 74. 86 k. J/mol ΔHf°(C(s, diamond)) = 1. 9 k. J/mol ΔHf°(HCl(g)) = – 92. 3 k. J/mol Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calculate the standard heat of reaction for the following: CH 4(g) + Cl 2(g) → C(s, diamond) + 4 HCl(g) ΔHf°(CH 4(g)) = – 74. 86 k. J/mol ΔHf°(C(s, diamond)) = 1. 9 k. J/mol ΔHf°(HCl(g)) = – 92. 3 k. J/mol ΔHf°(reactants) = [1 mol CH 4(g) ΔHf°CH 4(g)] + [1 mol Cl 2 ΔHf°Cl 2(g)] = – 74. 86 k. J + 0. 0 k. J = – 74. 86 k. J ΔHf°(products) = [1 mol C(s) ΔHf°C(s, diamond)] + [4 mol HCl ΔHf°HCl(g)] = 1. 9 k. J + (4 – 92. 3 k. J) = – 367. 3 k. J ΔH° = ΔHf°(products) – ΔHf°(reactants) = – 367. 3 k. J – (– 74. 86 k. J) = – 292. 4 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts & Key Equation Hess’s law allows you to determine the heat of reaction indirectly by using the known heats of reaction of two or more thermochemical equations. For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. ΔH° = ΔHf°(products) – ΔHf°(reactants) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • Hess’s law of heat summation: if you add two or more thermochemical equations to give a final equation, then you also add the heats of reaction to give the final heat of reaction • standard heat of formation (ΔHf°): the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25°C Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

BIG IDEA Matter and Energy The heat of reaction can be calculated by using the known heats of reaction of two or more thermochemical equations or by using standard heats of formation. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
 Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Thermochemistry form 3 exercise
Thermochemistry form 3 exercise Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
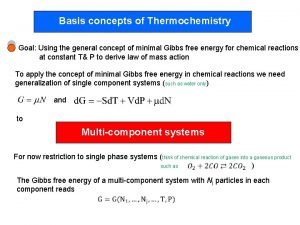
Chapter 17 thermochemistry Gibbs free energy unit
Gibbs free energy unit Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Nn
Nn Thermochemistry
Thermochemistry Thermochemistry gaussian
Thermochemistry gaussian Thermochemistry formulas
Thermochemistry formulas Cartoon thermochemistry
Cartoon thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry equations
Thermochemistry equations Symbolic border one pager
Symbolic border one pager Thermochemical equation
Thermochemical equation Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chemistry unit 7
Ap chemistry unit 7 Thermochemistry is the study of *
Thermochemistry is the study of * General chemistry thermochemistry
General chemistry thermochemistry Study of energy and its transformations
Study of energy and its transformations Ilmu kimia yang mempelajari tentang kalor reaksi
Ilmu kimia yang mempelajari tentang kalor reaksi Energy changes
Energy changes What is thermochemistry
What is thermochemistry Thermochemistry concepts
Thermochemistry concepts Unit 9 thermochemistry
Unit 9 thermochemistry Thermochemistry is study of
Thermochemistry is study of Energy diagram thermochemistry
Energy diagram thermochemistry Thermochemistry is concerned with the
Thermochemistry is concerned with the Kinetic energy thermochemistry
Kinetic energy thermochemistry Thermochemistry
Thermochemistry A piece of metal is heated then submerged
A piece of metal is heated then submerged Bomb calorimetry equation
Bomb calorimetry equation Thermochemistry video
Thermochemistry video Thermochemistry
Thermochemistry Thermochemistry
Thermochemistry Thermochemistry exam review
Thermochemistry exam review State and explain hess's law of constant heat summation.
State and explain hess's law of constant heat summation. Coffee cup calorimeter
Coffee cup calorimeter Q = m x cp x delta t
Q = m x cp x delta t How to calculate the heat absorbed
How to calculate the heat absorbed Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Thermochemistry vocabulary
Thermochemistry vocabulary Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là gì
Số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi 6 liters of oxygen is what percentage
6 liters of oxygen is what percentage Venturi mask
Venturi mask Venturi mask oxygen flow rate
Venturi mask oxygen flow rate Define streamline and turbulent flow
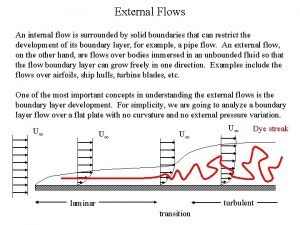
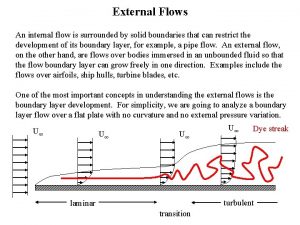
Define streamline and turbulent flow Internal and external flow
Internal and external flow Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Flow of energy vs flow of matter
Flow of energy vs flow of matter Structure chart in software engineering
Structure chart in software engineering Transform flow and transaction flow
Transform flow and transaction flow Differentiate between rotational and irrotational flow
Differentiate between rotational and irrotational flow Internal versus external flow
Internal versus external flow Data flow vs control flow
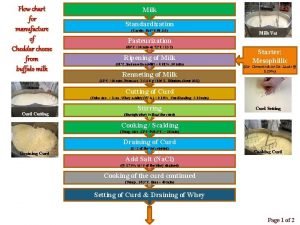
Data flow vs control flow Flow diagram of cheddar cheese
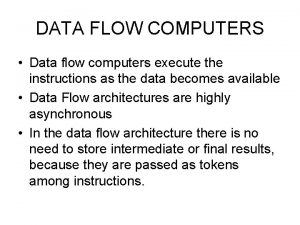
Flow diagram of cheddar cheese Control flow and data flow computers
Control flow and data flow computers Control flow vs transaction flow
Control flow vs transaction flow Chapter 4 lesson 2 energy flow in ecosystems
Chapter 4 lesson 2 energy flow in ecosystems Section 2 flow of energy in an ecosystem
Section 2 flow of energy in an ecosystem Flow chapter 13
Flow chapter 13 Chapter 4 lesson 2 energy flow in ecosystems answer key
Chapter 4 lesson 2 energy flow in ecosystems answer key Specific force in open channel flow
Specific force in open channel flow Steps in the flow of food
Steps in the flow of food Flow of food: preparation
Flow of food: preparation Chapter 6 discounted cash flow valuation
Chapter 6 discounted cash flow valuation Chapter 2 financial statements taxes and cash flow
Chapter 2 financial statements taxes and cash flow Closed conduits
Closed conduits Chapter one flow
Chapter one flow Chapter one flow
Chapter one flow Flow chapter 11
Flow chapter 11 The red tent book summary
The red tent book summary Great gatsby ch 8 summary
Great gatsby ch 8 summary Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Stoichiometry chapter 11 study guide
Stoichiometry chapter 11 study guide Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Chapter 7 similarity chapter test form a answer key
Chapter 7 similarity chapter test form a answer key Chapter 6 career readiness
Chapter 6 career readiness 7 ionic and metallic bonding
7 ionic and metallic bonding Chapter 9 surface water chapter assessment answer key
Chapter 9 surface water chapter assessment answer key Chapter 2 physics test prep representing motion
Chapter 2 physics test prep representing motion Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Chapter 7 ionic and metallic bonding chapter answer key
Chapter 7 ionic and metallic bonding chapter answer key Population ecology section 1 population dynamics answer key
Population ecology section 1 population dynamics answer key Chapter 2 standardized test practice answers
Chapter 2 standardized test practice answers Introduction to the book of philippians
Introduction to the book of philippians Ionic compound properties
Ionic compound properties 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems Tetraphosphorus octoxide formula
Tetraphosphorus octoxide formula Right triangle congruence theorems
Right triangle congruence theorems Wireless intelligent networking
Wireless intelligent networking Wide area work flow
Wide area work flow Wet processing flow chart
Wet processing flow chart Operation method sheet
Operation method sheet Basic flow of funds through the financial system
Basic flow of funds through the financial system Weka experimenter
Weka experimenter Nwrfc water supply
Nwrfc water supply Drainage basin inputs and outputs
Drainage basin inputs and outputs Horizontal flow grit chamber
Horizontal flow grit chamber Volunteer onboarding flow chart
Volunteer onboarding flow chart Flow of anions and cations in an electrochemical cell
Flow of anions and cations in an electrochemical cell Vlsi flow design
Vlsi flow design Interest tax shield calculation
Interest tax shield calculation Residual cash flow
Residual cash flow Why does efferent arteriole constriction increased gfr
Why does efferent arteriole constriction increased gfr Dwf and wwf
Dwf and wwf Uab comp clinic
Uab comp clinic Parallel heat exchanger
Parallel heat exchanger Stuck at fault
Stuck at fault Pressure-compensated flow control valve symbol
Pressure-compensated flow control valve symbol Flow specification
Flow specification Example of transaction flow graph
Example of transaction flow graph Water flow
Water flow Transaction flow graph
Transaction flow graph Data flow anomaly state graph
Data flow anomaly state graph Flsa exemption test flow chart
Flsa exemption test flow chart How does heat energy flow
How does heat energy flow 3 way cannula ppt
3 way cannula ppt Types of matter flow chart
Types of matter flow chart 2 step flow theory
2 step flow theory Leonardo da vinci turbulence
Leonardo da vinci turbulence Groundwater true/false quiz answers
Groundwater true/false quiz answers Bakki shower filter design
Bakki shower filter design Mdf process flow chart
Mdf process flow chart Effective green time
Effective green time Flow control udp
Flow control udp Sugar sink in plants
Sugar sink in plants Casparian strip
Casparian strip Auto attendant call flow diagram
Auto attendant call flow diagram Cpe wan
Cpe wan Flowbidlot
Flowbidlot Flow production examples
Flow production examples Electrons flow from anode to cathode
Electrons flow from anode to cathode Change in nwc equation
Change in nwc equation Mass flow rate formula
Mass flow rate formula Brace map example
Brace map example First law of thermodynamics for open system
First law of thermodynamics for open system Thermal mass flow meter calibration
Thermal mass flow meter calibration Fashion theories
Fashion theories What is the title of the map
What is the title of the map Where does the river shannon enter the sea
Where does the river shannon enter the sea Cerebrospinal fluid breathing
Cerebrospinal fluid breathing