Thermochemistry CHAPTER 17 Thermochemistry study of energy changes







- Slides: 48

Thermochemistry CHAPTER 17

Thermochemistry: study of energy changes During chemical reactions During phase changes

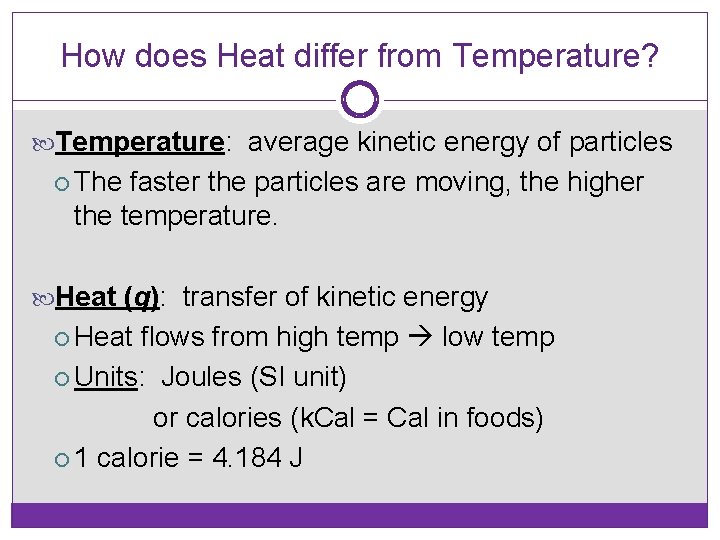
How does Heat differ from Temperature? Temperature: average kinetic energy of particles The faster the particles are moving, the higher the temperature. Heat (q): transfer of kinetic energy Heat flows from high temp low temp Units: Joules (SI unit) or calories (k. Cal = Cal in foods) 1 calorie = 4. 184 J

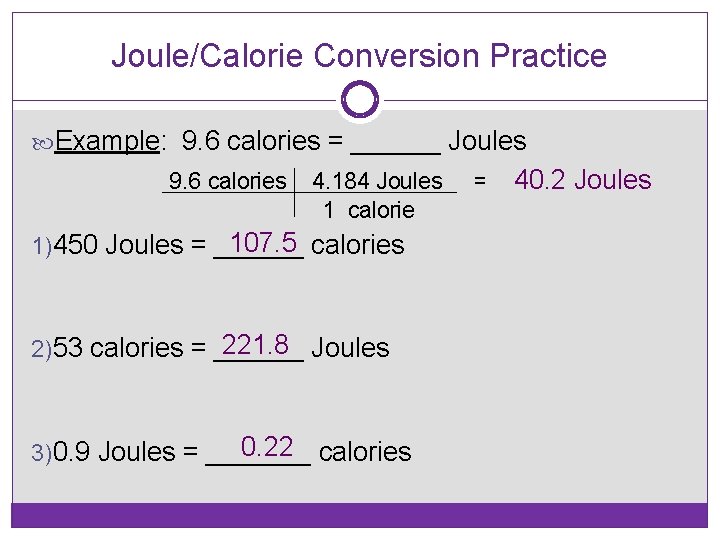
Joule/Calorie Conversion Practice Example: 9. 6 calories = ______ Joules 9. 6 calories 4. 184 Joules 1 calorie 107. 5 calories 1)450 Joules = ______ 221. 8 Joules 2)53 calories = ______ 0. 22 calories 3)0. 9 Joules = _______ = 40. 2 Joules

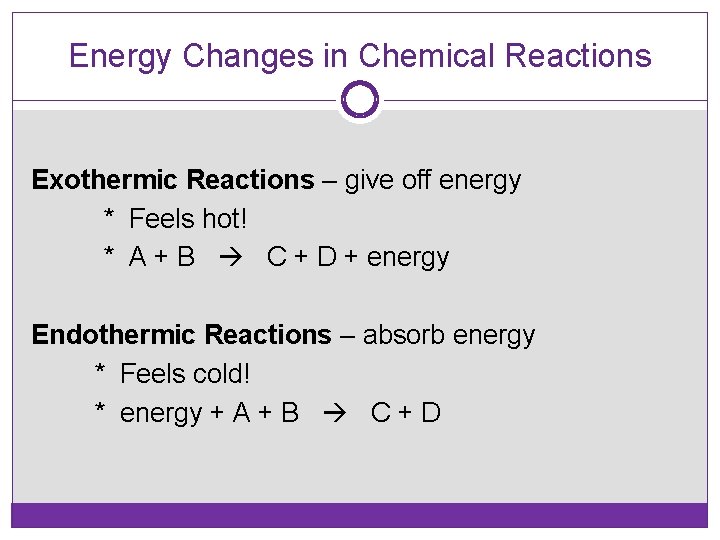
Energy Changes in Chemical Reactions Exothermic Reactions – give off energy * Feels hot! * A + B C + D + energy Endothermic Reactions – absorb energy * Feels cold! * energy + A + B C + D

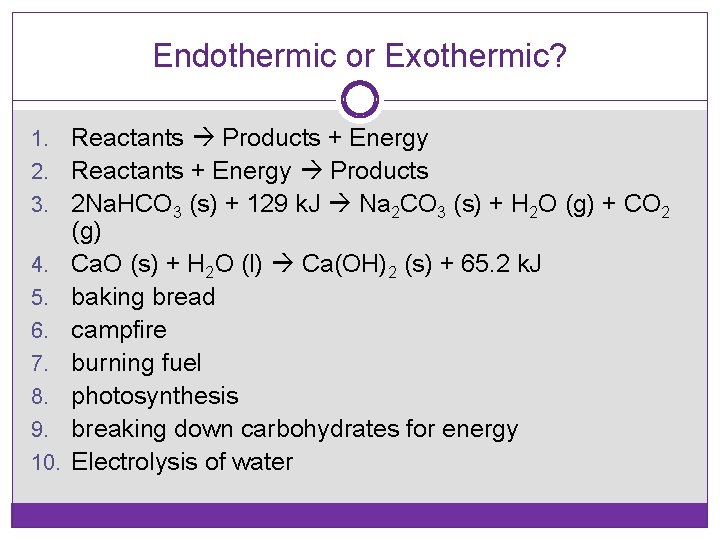
Endothermic or Exothermic? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Reactants Products + Energy Reactants + Energy Products 2 Na. HCO 3 (s) + 129 k. J Na 2 CO 3 (s) + H 2 O (g) + CO 2 (g) Ca. O (s) + H 2 O (l) Ca(OH)2 (s) + 65. 2 k. J baking bread campfire burning fuel photosynthesis breaking down carbohydrates for energy Electrolysis of water

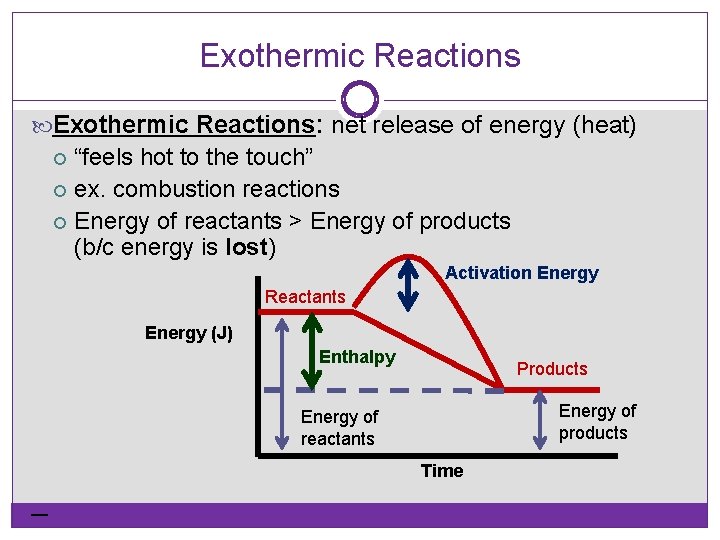
Exothermic Reactions: net release of energy (heat) “feels hot to the touch” ex. combustion reactions Energy of reactants > Energy of products (b/c energy is lost) Activation Energy Reactants Energy (J) Enthalpy Products Energy of products Energy of reactants Time

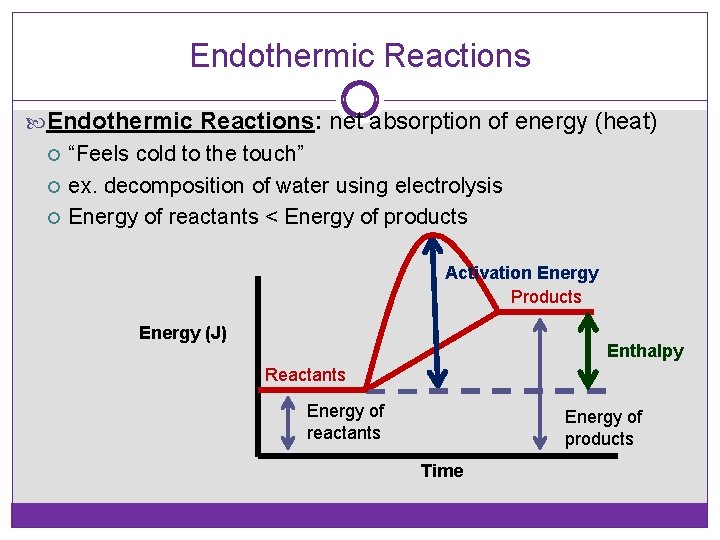
Endothermic Reactions: net absorption of energy (heat) “Feels cold to the touch” ex. decomposition of water using electrolysis Energy of reactants < Energy of products Activation Energy Products Energy (J) Enthalpy Reactants Energy of reactants Energy of products Time

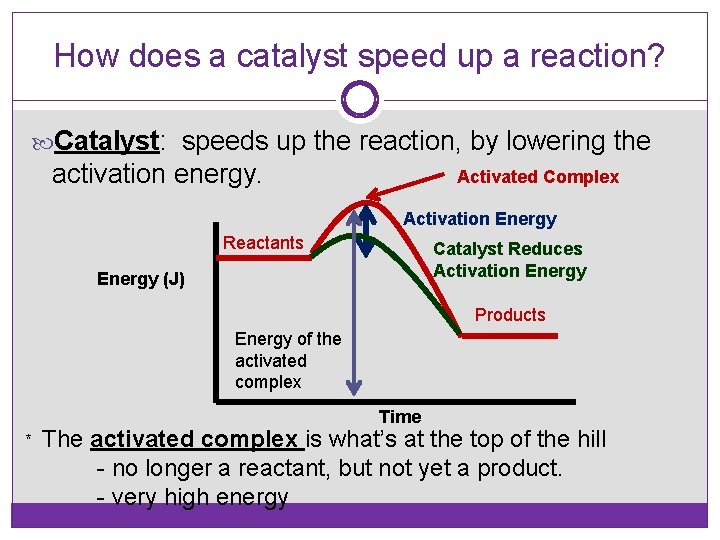
Activation Energy & Catalysts Activation energy: The amount of energy needed to get a reaction started. Required for BOTH endothermic and exothermic reactions.

How does a catalyst speed up a reaction? Catalyst: speeds up the reaction, by lowering the activation energy. Activated Complex Activation Energy Reactants Catalyst Reduces Activation Energy (J) Products Energy of the activated complex Time * The activated complex is what’s at the top of the hill - no longer a reactant, but not yet a product. - very high energy

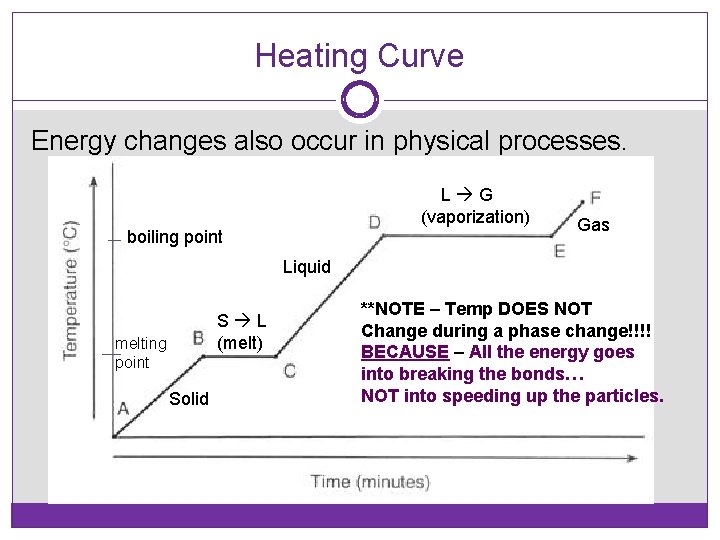
Heating Curve Energy changes also occur in physical processes. L G (vaporization) boiling point Gas Liquid S L (melt) melting point Solid **NOTE – Temp DOES NOT Change during a phase change!!!! BECAUSE – All the energy goes into breaking the bonds… NOT into speeding up the particles.

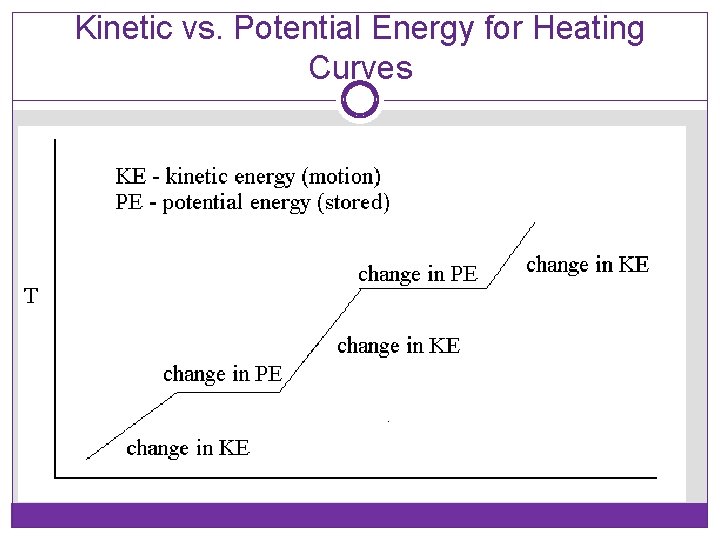
Kinetic vs. Potential Energy for Heating Curves

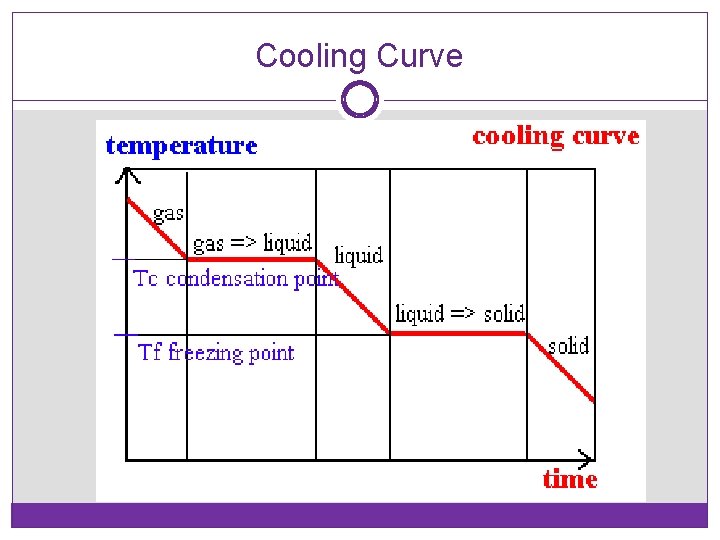
Cooling Curve

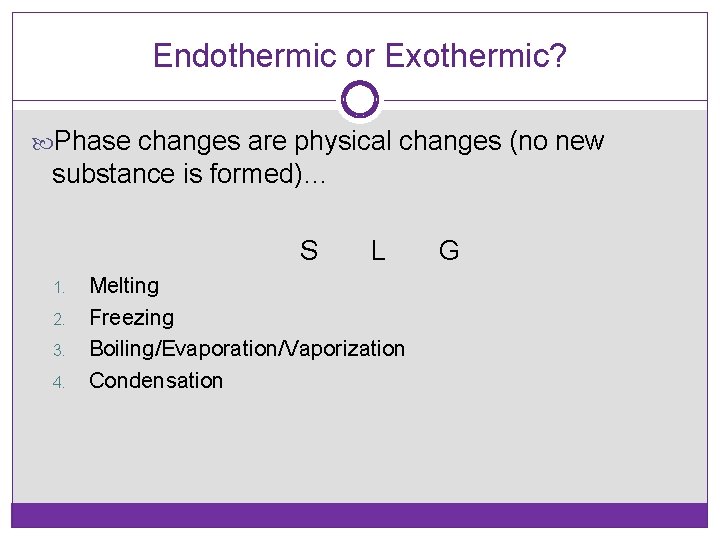
Endothermic or Exothermic? Phase changes are physical changes (no new substance is formed)… S 1. 2. 3. 4. L Melting Freezing Boiling/Evaporation/Vaporization Condensation G

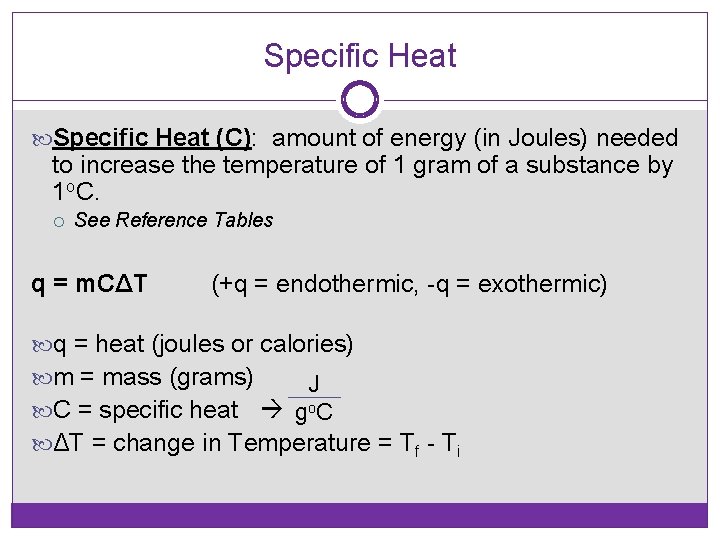
Specific Heat (C): amount of energy (in Joules) needed to increase the temperature of 1 gram of a substance by 1 o. C. See Reference Tables q = m. CΔT (+q = endothermic, -q = exothermic) q = heat (joules or calories) m = mass (grams) J C = specific heat go. C ΔT = change in Temperature = Tf - Ti

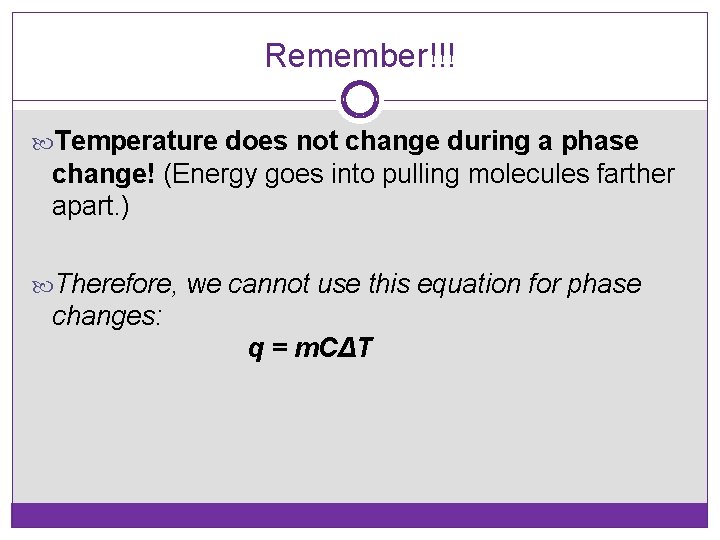
Remember!!! Temperature does not change during a phase change! (Energy goes into pulling molecules farther apart. ) Therefore, we cannot use this equation for phase changes: q = m. CΔT

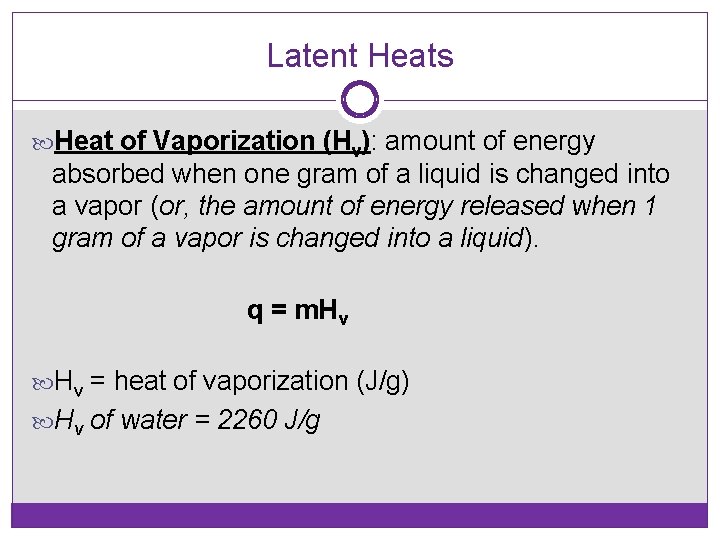
Latent Heats Heat of Vaporization (Hv): amount of energy absorbed when one gram of a liquid is changed into a vapor (or, the amount of energy released when 1 gram of a vapor is changed into a liquid). q = m. Hv = heat of vaporization (J/g) Hv of water = 2260 J/g

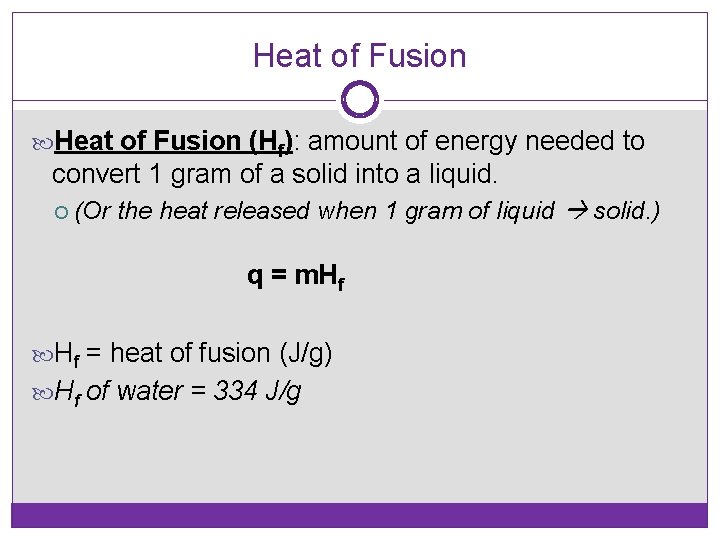
Heat of Fusion (Hf): amount of energy needed to convert 1 gram of a solid into a liquid. (Or the heat released when 1 gram of liquid solid. ) q = m. Hf Hf = heat of fusion (J/g) Hf of water = 334 J/g

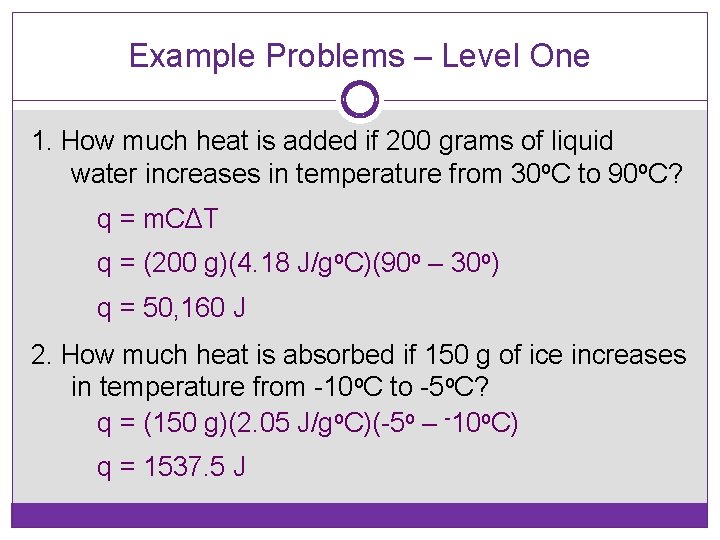
Example Problems – Level One 1. How much heat is added if 200 grams of liquid water increases in temperature from 30 o. C to 90 o. C? q = m. CΔT q = (200 g)(4. 18 J/go. C)(90 o – 30 o) q = 50, 160 J 2. How much heat is absorbed if 150 g of ice increases in temperature from -10 o. C to -5 o. C? q = (150 g)(2. 05 J/go. C)(-5 o – -10 o. C) q = 1537. 5 J

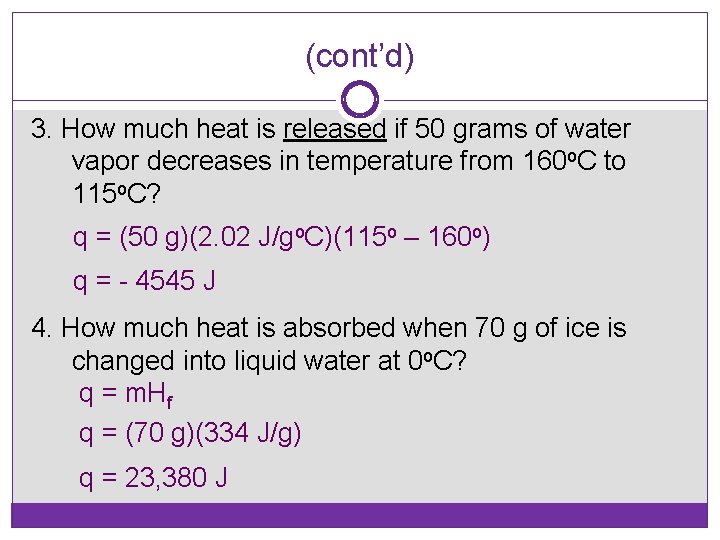
(cont’d) 3. How much heat is released if 50 grams of water vapor decreases in temperature from 160 o. C to 115 o. C? q = (50 g)(2. 02 J/go. C)(115 o – 160 o) q = - 4545 J 4. How much heat is absorbed when 70 g of ice is changed into liquid water at 0 o. C? q = m. Hf q = (70 g)(334 J/g) q = 23, 380 J

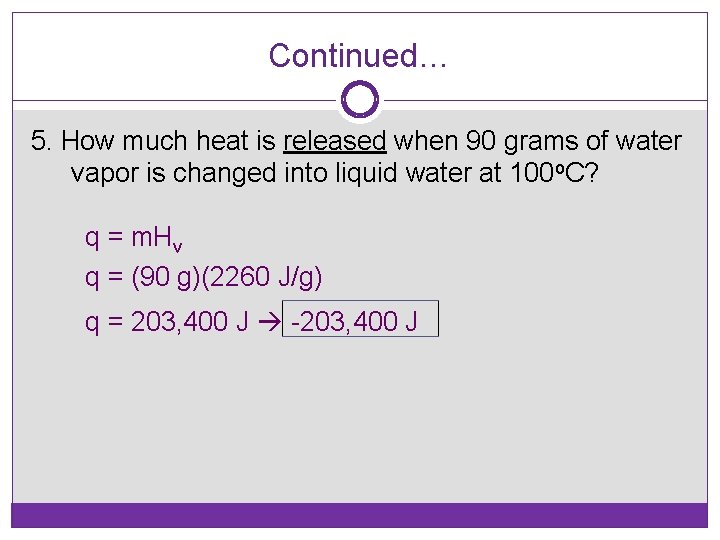
Continued… 5. How much heat is released when 90 grams of water vapor is changed into liquid water at 100 o. C? q = m. Hv q = (90 g)(2260 J/g) q = 203, 400 J -203, 400 J

Example Problems – Level Two 6. How much heat is absorbed if 55 grams of ice at -15 o. C is converted into liquid water? 2 steps: (1) heat the ice and (2) melt ice liquid (1) q = m. CΔT q = (55 g)(2. 05 J/go. C)(0 o – -15 o) q = 1691. 3 J (2) q = m. Hf q = (55 g)(334 J/g) q = 18, 370 J Add both steps together! Total heat = 1691. 3 J + 18, 370 J Total heat = 20, 061. 3 J

Continued… 7. How much heat is absorbed if 45 grams of water at 20 o. C is converted into steam at 110 o. C? 3 steps: (1) heat water, (2) water steam, (3) heat steam (1) q 1 = m. CΔT q 1 = (45 g)(4. 18 J/go. C)(100 o – 20 o) q 1 = 15, 048 J Add all 3 together! (2) q 2 = m. Hv qtotal = q 1 + q 2 + q 3 q 2 = (45 g)(2260 J/g) qtotal = 15, 048 J + 101, 700 J + 909 J q 2 = 101, 700 J qtotal = 117, 657 J (3) q 3 = m. CΔT q 3 = (45 g)(2. 02 J/go. C)(110 o – 100 o) q 3 = 909 J

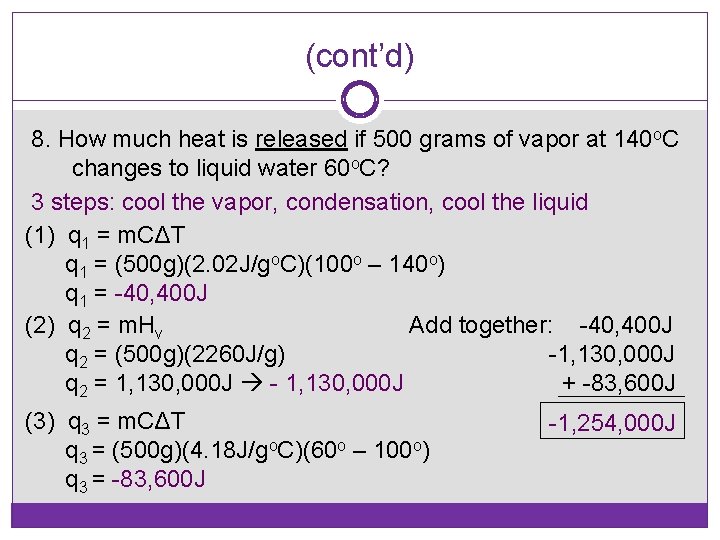
(cont’d) 8. How much heat is released if 500 grams of vapor at 140 o. C changes to liquid water 60 o. C? 3 steps: cool the vapor, condensation, cool the liquid (1) q 1 = m. CΔT q 1 = (500 g)(2. 02 J/go. C)(100 o – 140 o) q 1 = -40, 400 J (2) q 2 = m. Hv Add together: -40, 400 J q 2 = (500 g)(2260 J/g) -1, 130, 000 J q 2 = 1, 130, 000 J - 1, 130, 000 J + -83, 600 J (3) q 3 = m. CΔT q 3 = (500 g)(4. 18 J/go. C)(60 o – 100 o) q 3 = -83, 600 J -1, 254, 000 J

Calorimetry: the precise measurement of the heat flow out of a system for chemical and physical processes. - qreaction = qwater reaction info - m. C∆T = m. C∆T water info

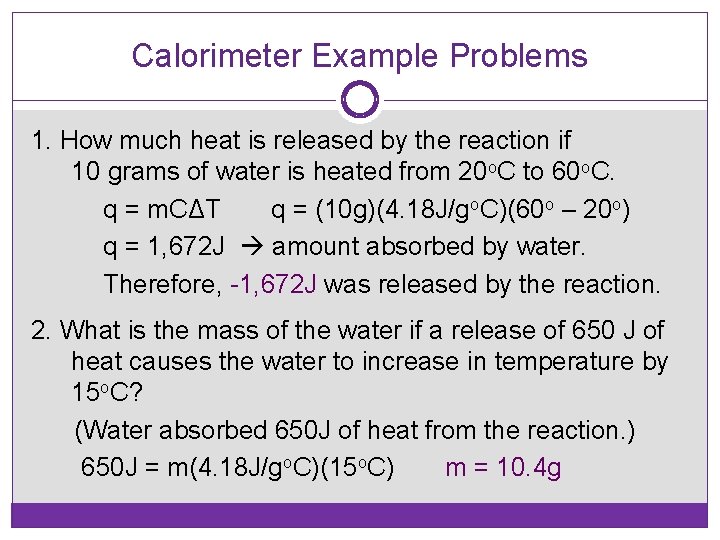
Calorimeter Example Problems 1. How much heat is released by the reaction if 10 grams of water is heated from 20 o. C to 60 o. C. q = m. CΔT q = (10 g)(4. 18 J/go. C)(60 o – 20 o) q = 1, 672 J amount absorbed by water. Therefore, -1, 672 J was released by the reaction. 2. What is the mass of the water if a release of 650 J of heat causes the water to increase in temperature by 15 o. C? (Water absorbed 650 J of heat from the reaction. ) 650 J = m(4. 18 J/go. C)(15 o. C) m = 10. 4 g

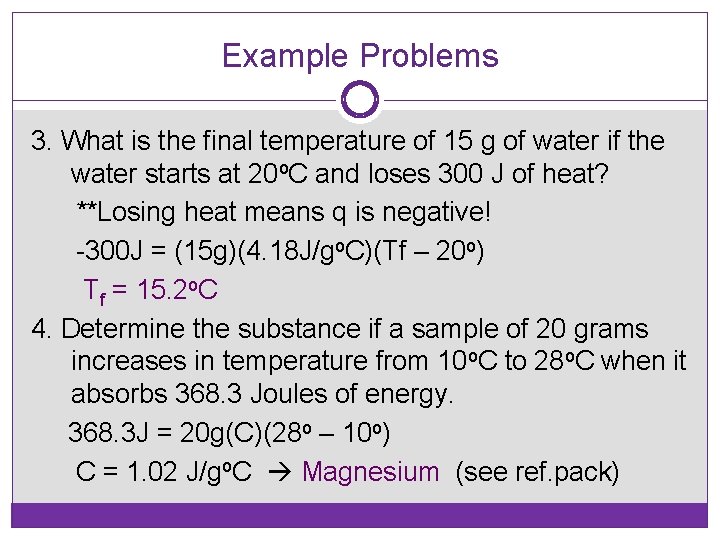
Example Problems 3. What is the final temperature of 15 g of water if the water starts at 20 o. C and loses 300 J of heat? **Losing heat means q is negative! -300 J = (15 g)(4. 18 J/go. C)(Tf – 20 o) Tf = 15. 2 o. C 4. Determine the substance if a sample of 20 grams increases in temperature from 10 o. C to 28 o. C when it absorbs 368. 3 Joules of energy. 368. 3 J = 20 g(C)(28 o – 10 o) C = 1. 02 J/go. C Magnesium (see ref. pack)

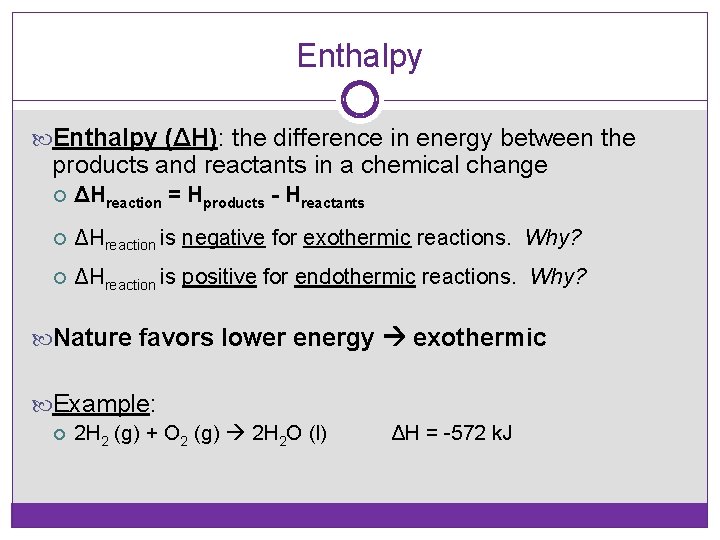
Enthalpy (ΔH): the difference in energy between the products and reactants in a chemical change ΔHreaction = Hproducts - Hreactants ΔHreaction is negative for exothermic reactions. Why? ΔHreaction is positive for endothermic reactions. Why? Nature favors lower energy exothermic Example: 2 H 2 (g) + O 2 (g) 2 H 2 O (l) ΔH = -572 k. J

Entropy (∆S): a measure of the disorder of the particles in a system. q Systems tend to become more disordered. Entropy increases when: q q q solid liquid gas (melting) # moles increase in a reaction (1 A + 2 B 3 C + 3 D) Temperature increases

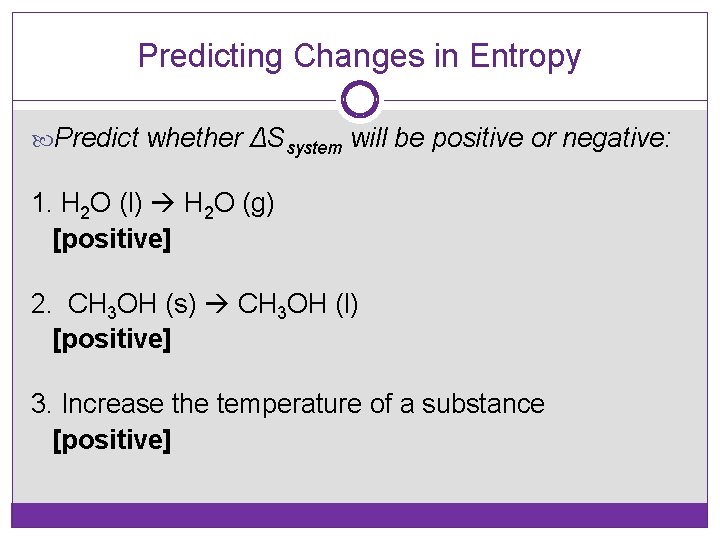
Predicting Changes in Entropy Predict whether ΔSsystem will be positive or negative: 1. H 2 O (l) H 2 O (g) [positive] 2. CH 3 OH (s) CH 3 OH (l) [positive] 3. Increase the temperature of a substance [positive]

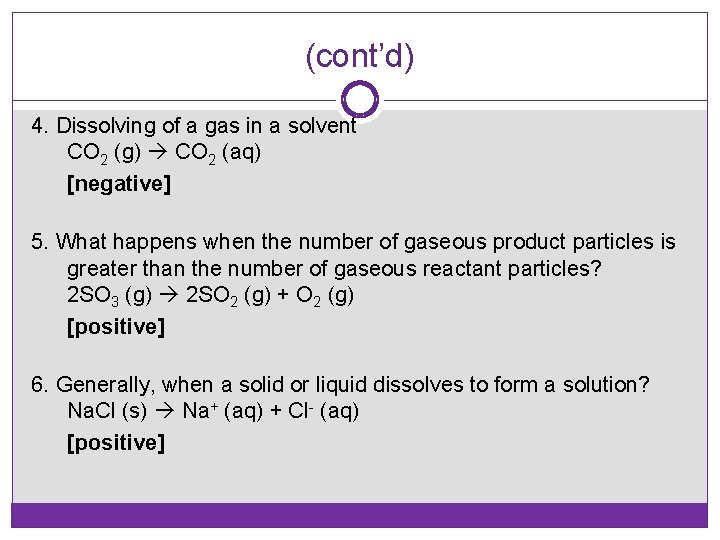
(cont’d) 4. Dissolving of a gas in a solvent CO 2 (g) CO 2 (aq) [negative] 5. What happens when the number of gaseous product particles is greater than the number of gaseous reactant particles? 2 SO 3 (g) 2 SO 2 (g) + O 2 (g) [positive] 6. Generally, when a solid or liquid dissolves to form a solution? Na. Cl (s) Na+ (aq) + Cl- (aq) [positive]

Spontaneous Reactions A reaction is spontaneous if: 1. Energy decreases (energy is released = exothermic) AND 2. Disorder increases (positive entropy). Ex. Wood burning


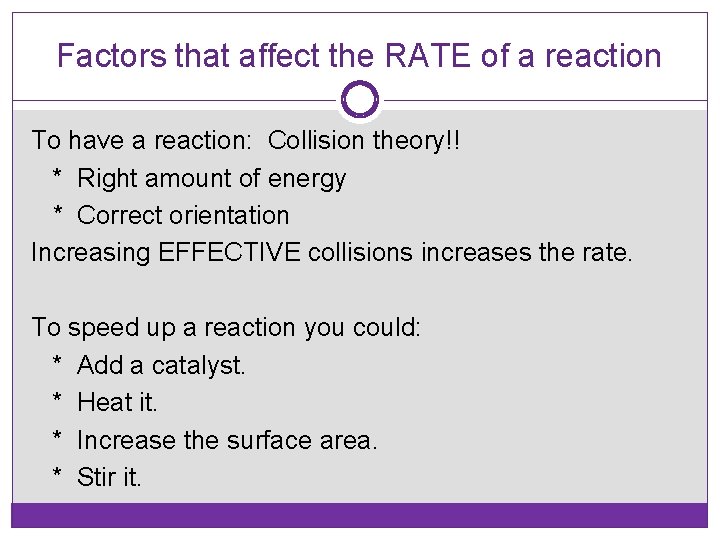
Factors that affect the RATE of a reaction To have a reaction: Collision theory!! * Right amount of energy * Correct orientation Increasing EFFECTIVE collisions increases the rate. To speed up a reaction you could: * Add a catalyst. * Heat it. * Increase the surface area. * Stir it.

Reaction Rate Cartoon http: //www. youtube. com/watch? v=Ott. RV 5 yk. P 7 A

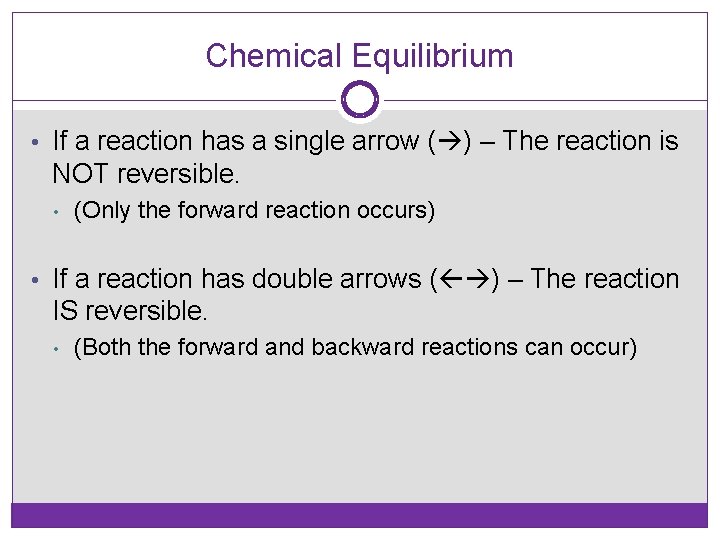
Chemical Equilibrium • If a reaction has a single arrow ( ) – The reaction is NOT reversible. • (Only the forward reaction occurs) • If a reaction has double arrows ( ) – The reaction IS reversible. • (Both the forward and backward reactions can occur)

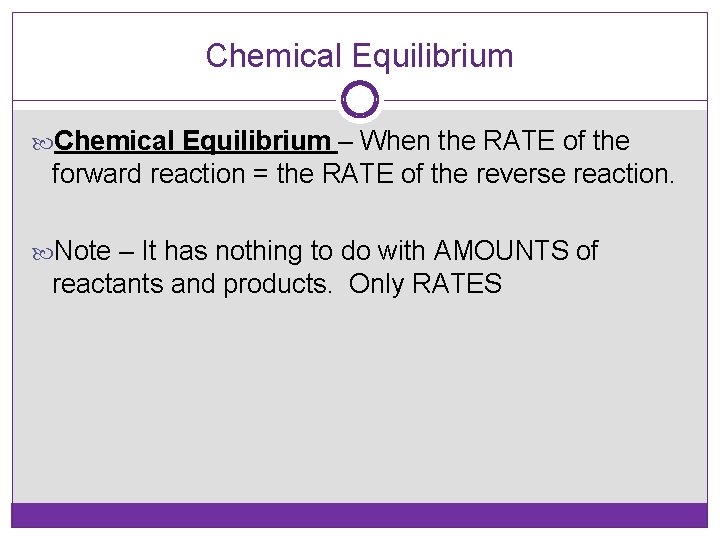
Chemical Equilibrium – When the RATE of the forward reaction = the RATE of the reverse reaction. Note – It has nothing to do with AMOUNTS of reactants and products. Only RATES

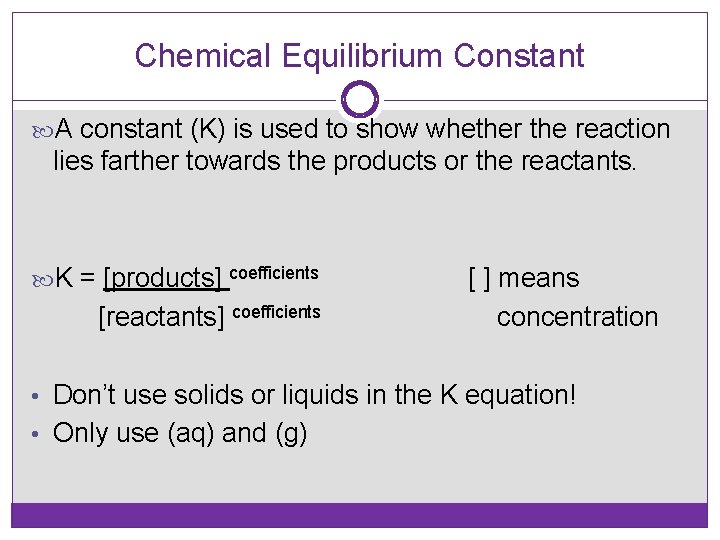
Chemical Equilibrium Constant A constant (K) is used to show whether the reaction lies farther towards the products or the reactants. K = [products] coefficients [reactants] coefficients [ ] means concentration • Don’t use solids or liquids in the K equation! • Only use (aq) and (g)

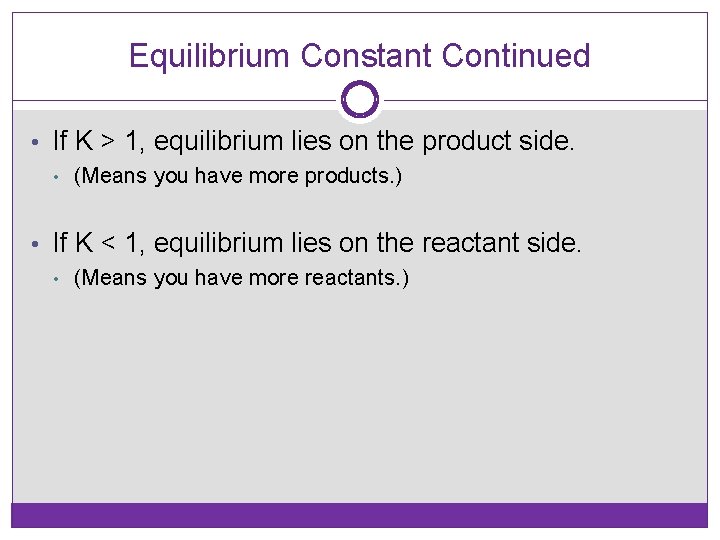
Equilibrium Constant Continued • If K > 1, equilibrium lies on the product side. • (Means you have more products. ) • If K < 1, equilibrium lies on the reactant side. • (Means you have more reactants. )

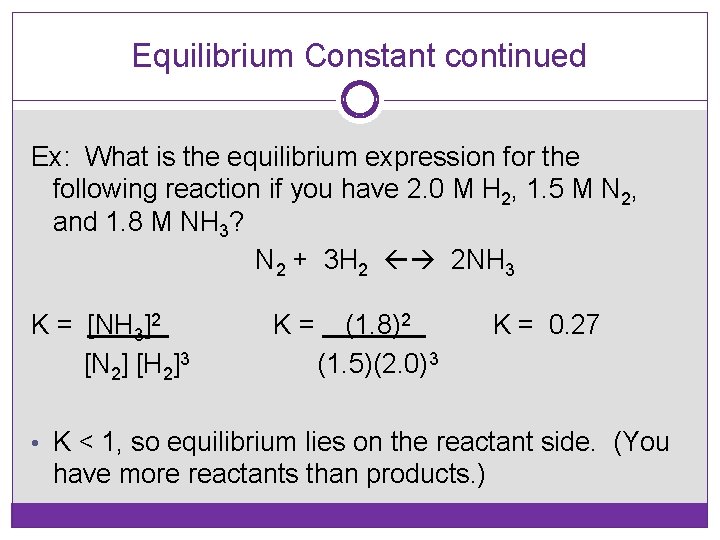
Equilibrium Constant continued Ex: What is the equilibrium expression for the following reaction if you have 2. 0 M H 2, 1. 5 M N 2, and 1. 8 M NH 3? N 2 + 3 H 2 2 NH 3 K = [NH 3]2 [N 2] [H 2]3 K= (1. 8)2 (1. 5)(2. 0)3 K = 0. 27 • K < 1, so equilibrium lies on the reactant side. (You have more reactants than products. )

Le. Chatelier’s Principle – When equilibrium is stressed, the reaction will shift to relieve the stress and restore equilibrium. There are three ways a reaction can be “stressed”. Change the amount of products or reactants. Change the pressure. Change the temperature.

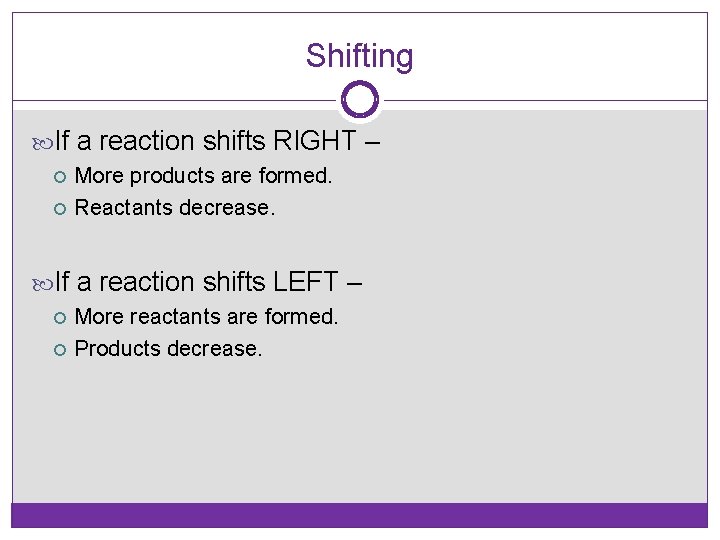
Shifting If a reaction shifts RIGHT – More products are formed. Reactants decrease. If a reaction shifts LEFT – More reactants are formed. Products decrease.

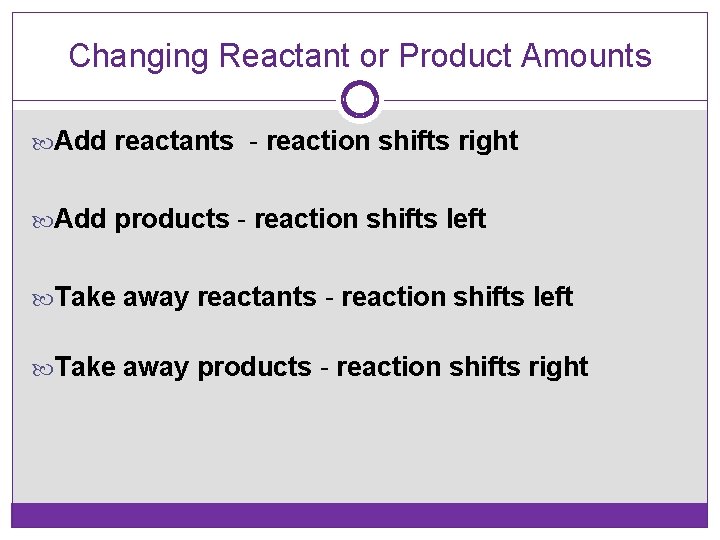
Changing Reactant or Product Amounts Add reactants - reaction shifts right Add products - reaction shifts left Take away reactants - reaction shifts left Take away products - reaction shifts right

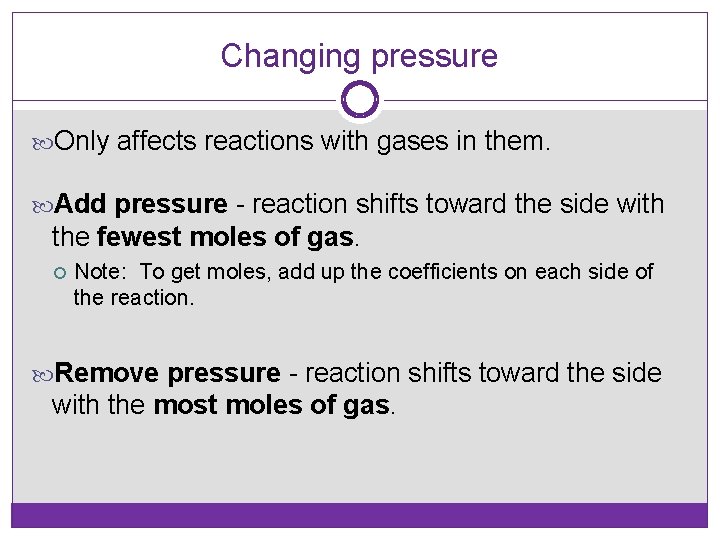
Changing pressure Only affects reactions with gases in them. Add pressure - reaction shifts toward the side with the fewest moles of gas. Note: To get moles, add up the coefficients on each side of the reaction. Remove pressure - reaction shifts toward the side with the most moles of gas.

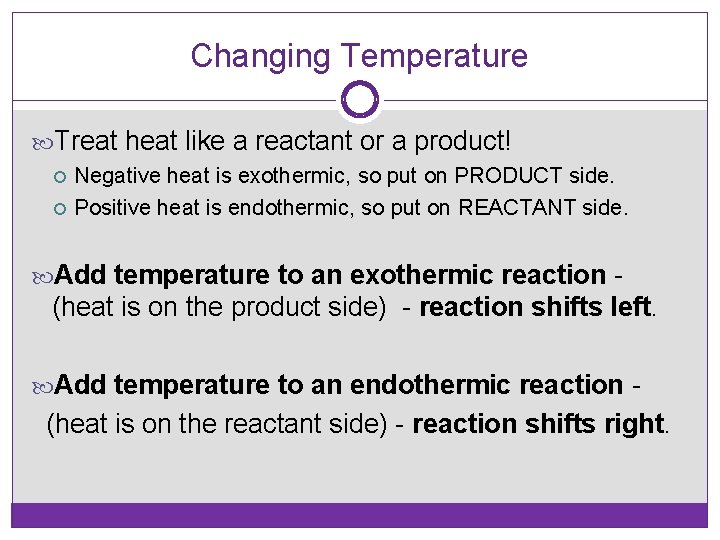
Changing Temperature Treat heat like a reactant or a product! Negative heat is exothermic, so put on PRODUCT side. Positive heat is endothermic, so put on REACTANT side. Add temperature to an exothermic reaction - (heat is on the product side) - reaction shifts left. Add temperature to an endothermic reaction - (heat is on the reactant side) - reaction shifts right.

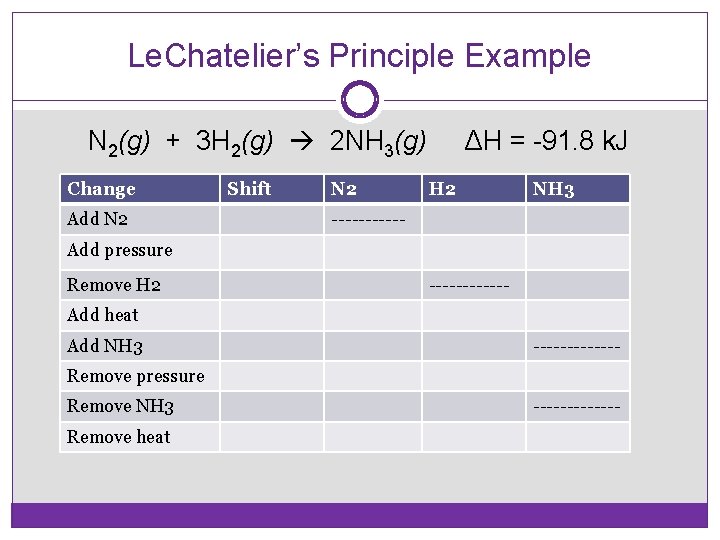
Le. Chatelier’s Principle Example N 2(g) + 3 H 2(g) 2 NH 3(g) Change Add N 2 Shift N 2 ΔH = -91. 8 k. J H 2 NH 3 ------ Add pressure Remove H 2 ------ Add heat Add NH 3 ------- Remove pressure Remove NH 3 Remove heat -------

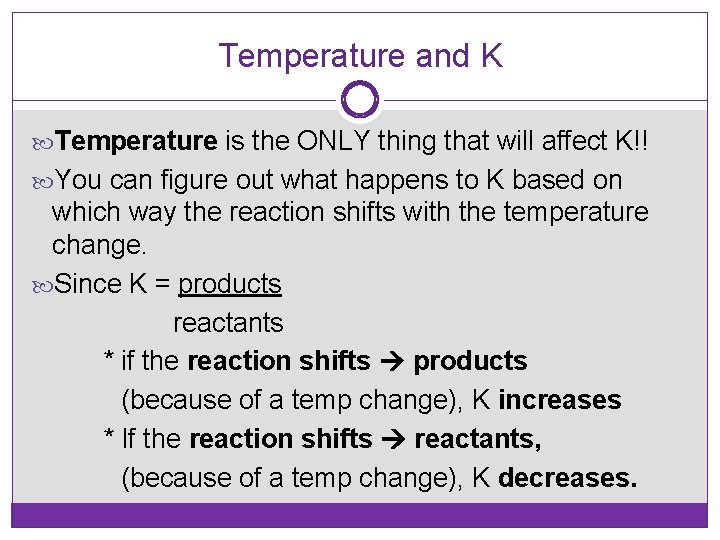
Temperature and K Temperature is the ONLY thing that will affect K!! You can figure out what happens to K based on which way the reaction shifts with the temperature change. Since K = products reactants * if the reaction shifts products (because of a temp change), K increases * If the reaction shifts reactants, (because of a temp change), K decreases.

 Changes in latitudes, changes in attitudes meaning
Changes in latitudes, changes in attitudes meaning Examples of physical change
Examples of physical change Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is study of
Thermochemistry is study of Thermochemistry is the study of
Thermochemistry is the study of Study of energy transformations
Study of energy transformations Ilmu kimia yang mempelajari kalor adalah
Ilmu kimia yang mempelajari kalor adalah Thermochemistry is study of
Thermochemistry is study of Thermochemistry
Thermochemistry Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chemical change
Chemical change A researcher decides to study cognitive changes
A researcher decides to study cognitive changes Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Science form 3 chapter 5 thermochemistry
Science form 3 chapter 5 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry What is delta h
What is delta h Energy changes
Energy changes Energy changes
Energy changes What is thermochemistry
What is thermochemistry Chapter 12 thermal energy
Chapter 12 thermal energy Chapter 11 study guide energy and its conservation answers
Chapter 11 study guide energy and its conservation answers Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Equation analysis sheet
Equation analysis sheet Chapter 10 geriatric care
Chapter 10 geriatric care Counterbalancing example
Counterbalancing example Chapter 5 section 3 changes in supply
Chapter 5 section 3 changes in supply Chapter 3 analyzing changes in financial position answers
Chapter 3 analyzing changes in financial position answers Chapter 21 revolutionary changes in the atlantic world
Chapter 21 revolutionary changes in the atlantic world Chapter 5 section 3 changes in supply
Chapter 5 section 3 changes in supply Chapter 3 analyzing changes in financial position answers
Chapter 3 analyzing changes in financial position answers Counterbalancing example
Counterbalancing example Changes in the land chapter 5 summary
Changes in the land chapter 5 summary Chapter 6 section 2 changes in market equilibrium
Chapter 6 section 2 changes in market equilibrium Thermochemistry
Thermochemistry Thermochemical equation
Thermochemical equation Thermochemistry
Thermochemistry Gaussian thermochemistry
Gaussian thermochemistry Thermochemical equation
Thermochemical equation Thermochemistry clipart
Thermochemistry clipart Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry equations
Thermochemistry equations