Thermochemistry Thermochemistry n The study of the changes






































- Slides: 83

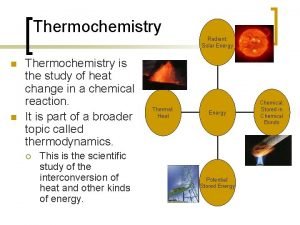
Thermochemistry

Thermochemistry** n The study of the changes in heat energy that accompany chemical reactions and physical changes.

As a group n On your index card Define: n n n Heat Temperature Thermal Equilibrium

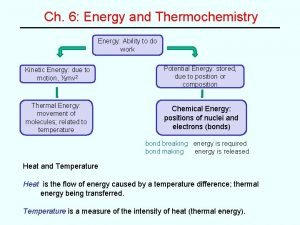
Heat** n n a form of energy (properly termed internal energy) depends on the amt of motion increases as the particles move faster total KE of the particles n ex. rub hands together (quickly), slide down rope quickly (rope burn)

Heat vs. Temperature n How do you detect internal energy? n temperature allows us to detect heat HEAT ≠ TEMPERATURE (are related)

Temperature** n n n measure of the average KE of molecules increases the faster the molecules are moving thermometer is instrument Mercury red alcohol temp scales o F, o. C and K

Thermal Equilibrium Soda

Thermal Equilibrium** n n state in which two bodies in contact with each other have identical temperatures basis for measuring temperature with thermometers

Heat n n **Heat cannot be measured directly – only indirectly by in temp. a change in temperature indicates the transfer of energy between substances by heat.

Heat n the transfer of energy between objects at different temps. n n an increase in temp. indicates the addition of energy a decrease in temp. indicates the removal of energy **Symbol Q **Joule(SI) or calorie (common for food) = unit to measure heat n n How is the energy of food listed? Note the unit for cal= C 1 Calorie = 1000 calories = 1 kcal 1 cal = 4. 186 J calorimeter is instrument used to “measure” heat

Heat n Calorimetry is used to determine the heat released or absorbed in a chemical reaction. The calorimeters shown here can determine the heat of a solution reaction at constant (atmospheric) pressure.

n Does the addition of heat insure an increase in temperature? No, a phase change is possible. If heat is removed does that insure that the temperature decreases? No, a phase change is possible.

Questions to consider? n Why would food be measured in “C” calories?

Questions to consider? n Why does your mother make you keep thermometer in you mouth for at least 3 mins. ? .

I need a volunteer… n n n Heat Temperature Thermal equilibrium

Questions to consider? n n Why do we put warm drinks into ice? How does the ice cool the drinks? Soda

Ice n n Predict the temperature of the ice? Is this possible? Why?

Ouch!!

Ice n Make a prediction as to the temperature that this ice will melt. (ie. If placed on a hot plate will it begin melting immediately? )

Internal Energy Changes n When a substance is heated, the energy of its particles is increased.

Internal Energy Changes n If the potential energy changes the physical state of the substance will change. if Potential Energy increases: s l, l g, or s g

Internal Energy Changes n If the kinetic energy increases the temperature of the substance increases.


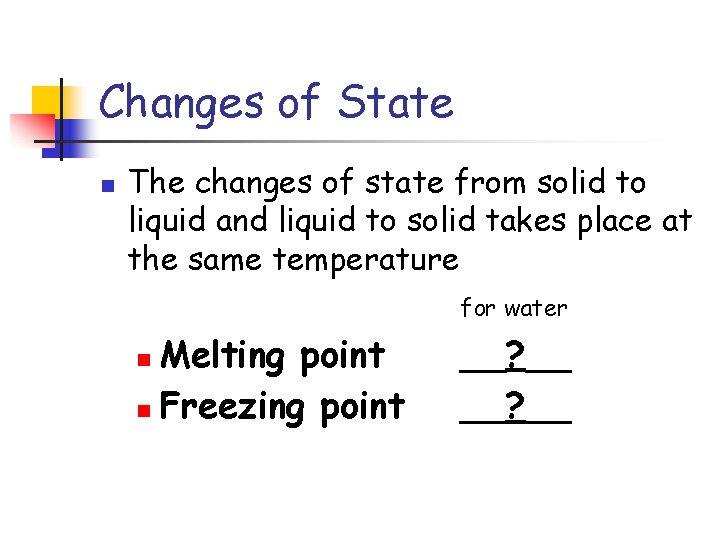
Changes of State n The changes of state from solid to liquid and liquid to solid takes place at the same temperature for water Melting point n Freezing point n __? __

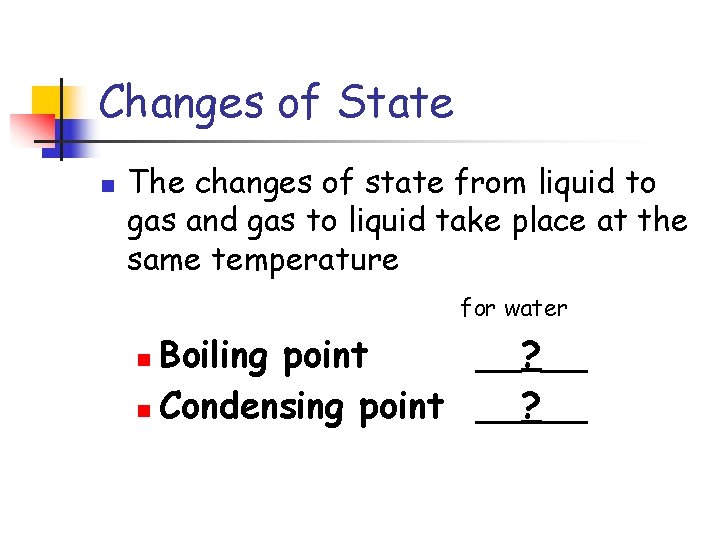
Changes of State n The changes of state from liquid to gas and gas to liquid take place at the same temperature for water Boiling point __? __ n Condensing point __? __ n


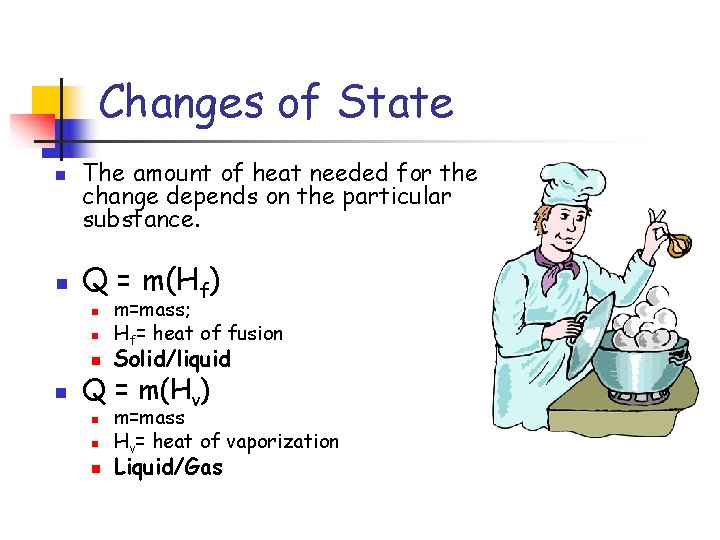
Changes of State n n The amount of heat needed for the change depends on the particular substance. Q = m(Hf) n n m=mass; Hf= heat of fusion Solid/liquid Q = m(Hv) n n n m=mass Hv= heat of vaporization Liquid/Gas

Questions to Consider? n When food was stored in cellars, during the winter, people would often place an open barrel of water in the cellar alongside their produce. Explain why this was done and why it would be effective.

Phase Change Equations** n Q = m(Hf) n n Hf = 334 J/g for water** Q = m(Hv) n Hv = 2260 J/g for water** Make sure units cancel !!!

Sample problems n n Determine the energy change involved in converting 16. 2 grams of ice to liquid water, both at 0 o. C. Q = (16. 2 g)(334 J/g) = 5410 J energy is absorbed

Sample problems n n Determine the energy change involved in converting 5. 8 grams of water to steam, both at 100 o. C. Q = (5. 8 g)(2260 J/g) = 1. 3 E 4 J energy is absorbed Why does it require so much more energy to go to a gas?

Sample problems n Determine the energy change involved to: n n Convert 98. 2 grams of water to ice, both at 0 o. C. Convert 52. 6 grams of steam to water, both at 100 o. C.

Sample problems n Determine the energy change involved to: n Convert 98. 2 grams of water to ice at 0 o. C. n Q=(98. 2 g)(334 J/g) = 32800 J n energy is released = -32800 J

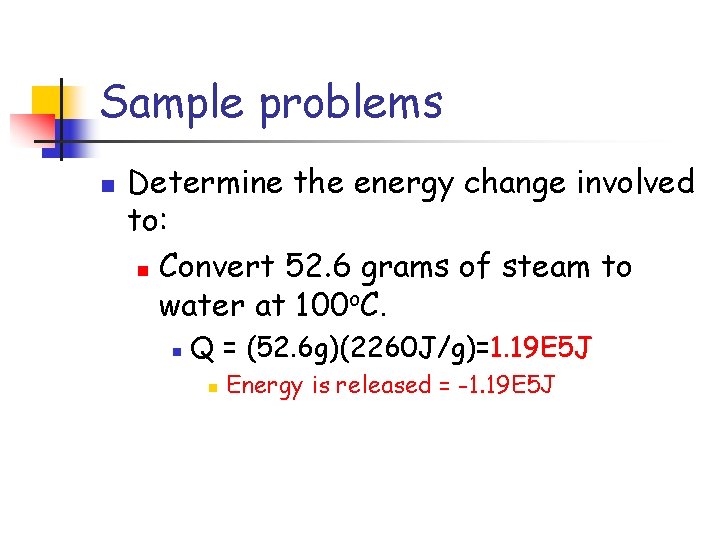
Sample problems n Determine the energy change involved to: n Convert 52. 6 grams of steam to water at 100 o. C. n Q = (52. 6 g)(2260 J/g)=1. 19 E 5 J n Energy is released = -1. 19 E 5 J

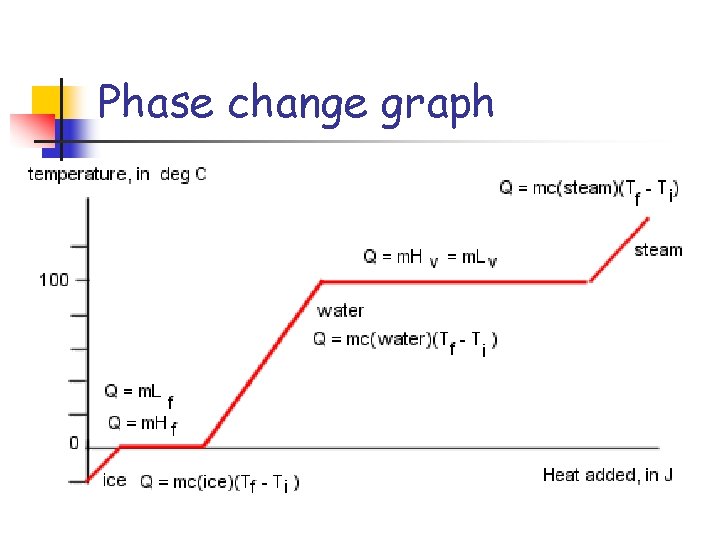
Phase change graph

When temperature does change n Frequently when energy is added to a substance the temperature does increase.

Questions to consider? n Can you give me an example of two things which are exposed to the same energy, yet have different temperatures?

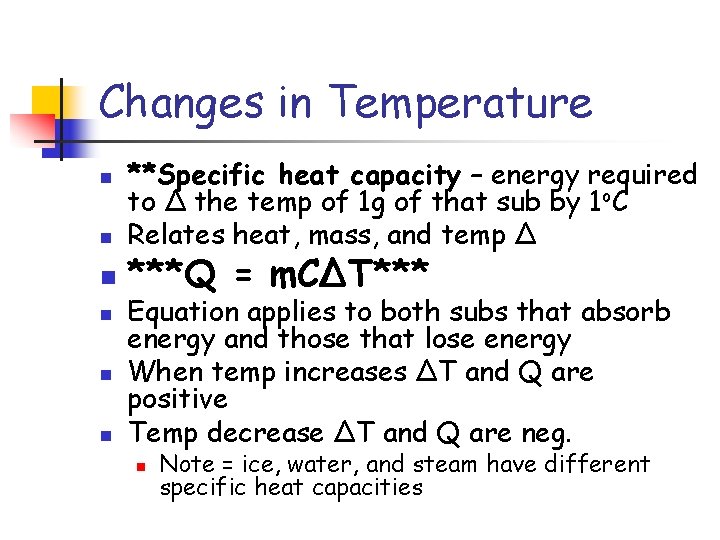
Changes in Temperature n n n **Specific heat capacity – energy required to Δ the temp of 1 g of that sub by 1 o. C Relates heat, mass, and temp Δ ***Q = m. CΔT*** Equation applies to both subs that absorb energy and those that lose energy When temp increases ΔT and Q are positive Temp decrease ΔT and Q are neg. n Note = ice, water, and steam have different specific heat capacities

Sample problems n Hypothermia can occur if the body temperature drops to 35. 0°C, although people have been known to survive much lower temperatures. On January 19, 1985, 2 -year-old Michael Trode was found in the snow near his Milwaukee home with a body temperature of 16. 0°C. If Michael's mass was 10. 0 kg, how much heat did his body lose, assuming his normal body temperature was 37. 0°C? (Happily, Michael survived!) Chuman body =3. 47 J/g°C

Sample problems Q= (10 000 g)(3. 47 J/g°C)(16. 0°C - 37. 0°C ) = - 728700 J = - 7. 29 E 5 J

Sample problems n Determine the energy required (in kilojoules) when cooling 456. 2 grams of water at 89. 2 °C to a final temperature of 5. 9 °C

Sample problems n n Determine the energy required (in kilojoules) when cooling 456. 2 grams of water at 89. 2 °C to a final temperature of 5. 9 °C Q =(456. 2 g)(4. 18 J/g°C)(5. 9°C-89. 2°C) = - 158846. 1 J = - 1. 59 E 5 J

Sample problem n Determine the energy released when converting 500. 0 g of ice at -25. 0 °C to steam at 110. 0 °C.

Sample problem: Steps 1. 2. 3. 4. 5. Heat solid to melting point. Melt solid. Heat liquid to boiling point. Boil liquid. Heat gas to required temperature.

Sample problem: Steps n n n Q = (500. 0 g)(2. 05 J/go. C)(0 - -25. 0 o. C) 25625 J Q = (500. 0 g)(334 J/g) 167000 J Q =(500. 0 g)(4. 18 J/go. C)(100. 0 o. C-0 o. C) 209000 J = 2. 090 E 5 n n Q =(500. 0 g)(2260 J/g) 1130000 J Q =(500. 0 g)(2. 02 J/go. C)(110. 0 o. C-100. 0 o. C) 10100 J

Sample problem: Steps Q = 25625 J + 167000 J + 209000 J + 1130000 J + 10100 J Q = 1541725 J =1542000 J (thousands is least significant)


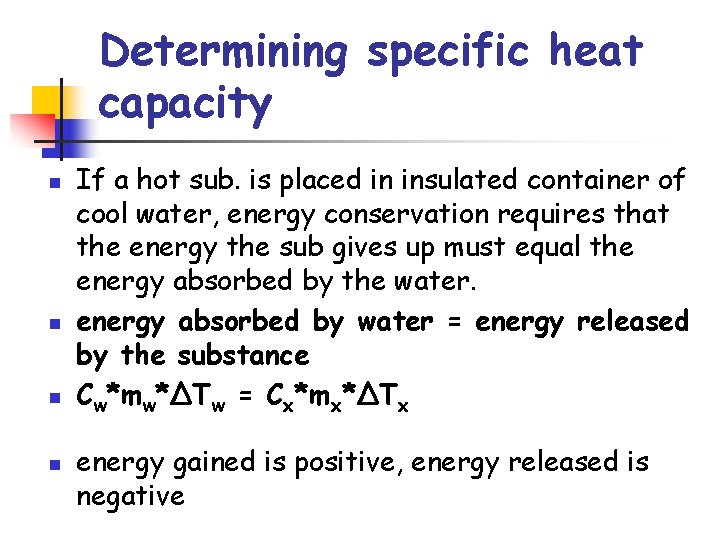
Determining specific heat capacity n n If a hot sub. is placed in insulated container of cool water, energy conservation requires that the energy the sub gives up must equal the energy absorbed by the water. energy absorbed by water = energy released by the substance Cw*mw*ΔTw = Cx*mx*ΔTx energy gained is positive, energy released is negative

Sample problem n Emily is testing her baby's bath water and finds that it is too cold, so she adds some hot water from a kettle on the stove. If Emily adds 2. 00 kg of water at 80. 0°C to 20. 0 kg of bath water at 27. 0°C, what is the final temperature of the bath water?

Sample problem n n Emily is testing her baby's bath water and finds that it is too cold, so she adds some hot water from a kettle on the stove. If Emily adds 2. 00 kg of water at 80. 0°C to 20. 0 kg of bath water at 27. 0°C, what is the final temperature of the bath water? (2000 g)(4. 18 J/g°C)(Tf-80. 0°C) = (20000 g)(4. 18 J/g°C)(Tf -27. 0°C)

Sample problem - (2000 g)(4. 18 J/g°C)(Tf-80. 0°C)=(20000 g)(4. 18 J/g°C)(Tf -27. 0°C) -2000 g. Tf – (-160000 go. C) = 20000 g. Tf – 540000 go. C +2000 g. Tf +540000 go. C 700000 go. C = 22000 g. Tf 22000 g = 22000 g 31. 8 o. C = Tf

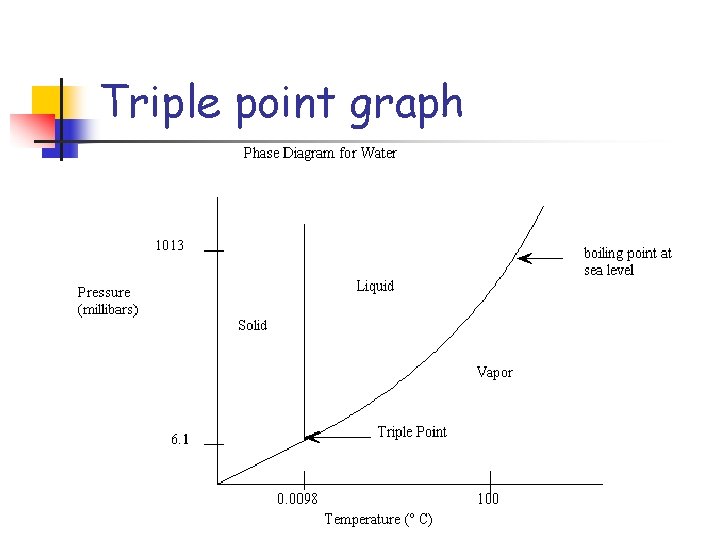
Pressure and phase change n Not only can temperature changes cause phase changes, but pressure changes can also cause phase changes.

Triple point graph

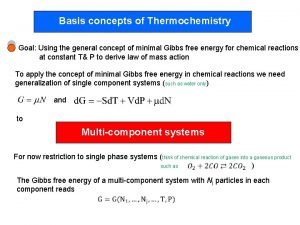
Heat of Reaction

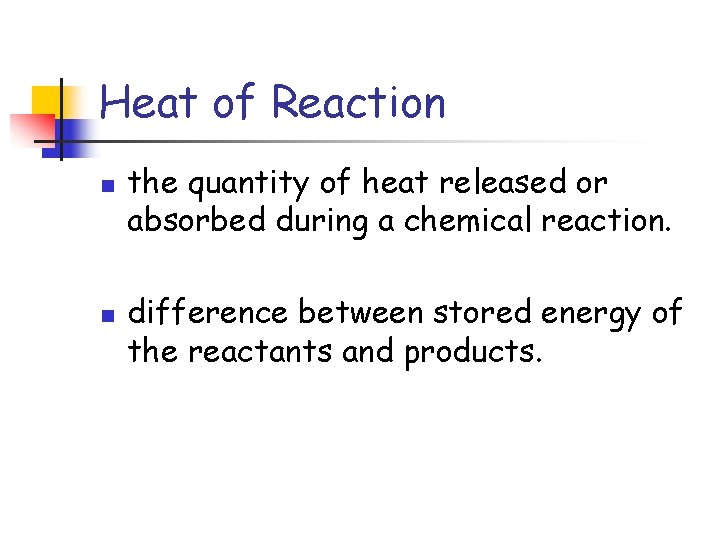
Heat of Reaction n n the quantity of heat released or absorbed during a chemical reaction. difference between stored energy of the reactants and products.

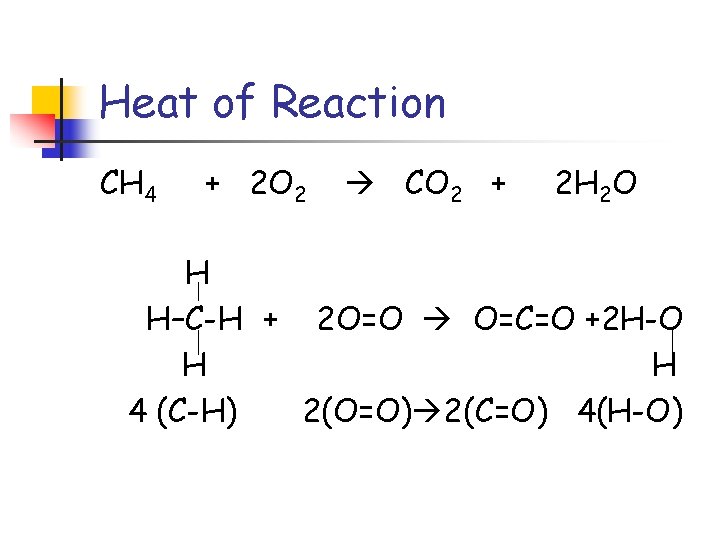
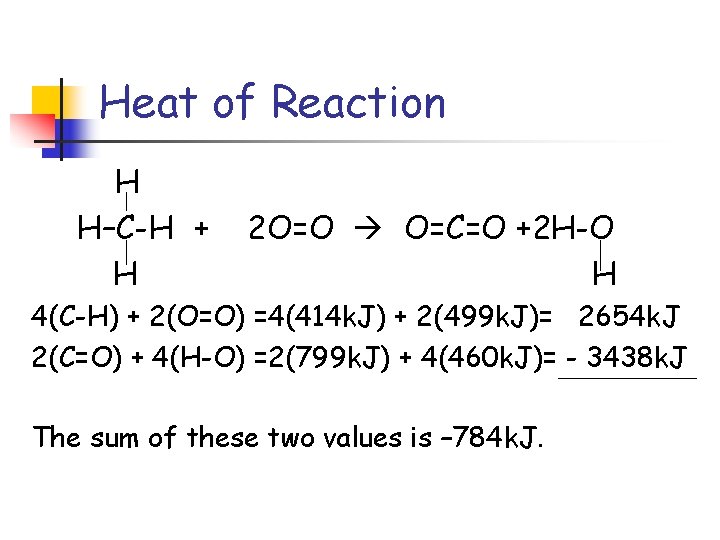
Heat of Reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O H H–C-H + 2 O=O O=C=O +2 H-O H H 4 (C-H) 2(O=O) 2(C=O) 4(H-O)

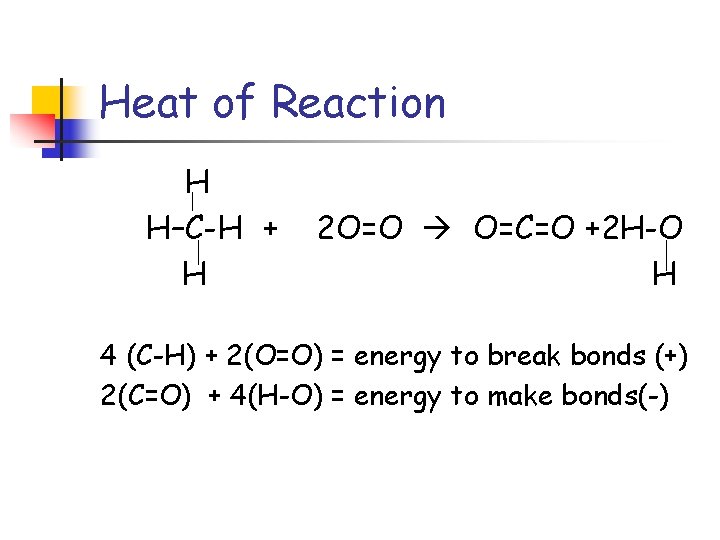
Heat of Reaction H H–C-H + H 2 O=O O=C=O +2 H-O H 4 (C-H) + 2(O=O) = energy to break bonds (+) 2(C=O) + 4(H-O) = energy to make bonds(-)

Heat of Reaction H H–C-H + H 2 O=O O=C=O +2 H-O H 4(C-H) + 2(O=O) =4(414 k. J) + 2(499 k. J)= 2654 k. J 2(C=O) + 4(H-O) =2(799 k. J) + 4(460 k. J)= - 3438 k. J The sum of these two values is – 784 k. J.

Heat of reaction n While bond energy calculations give an estimate of heats of reaction; it is possible to measure the energy change during a reaction in the lab.

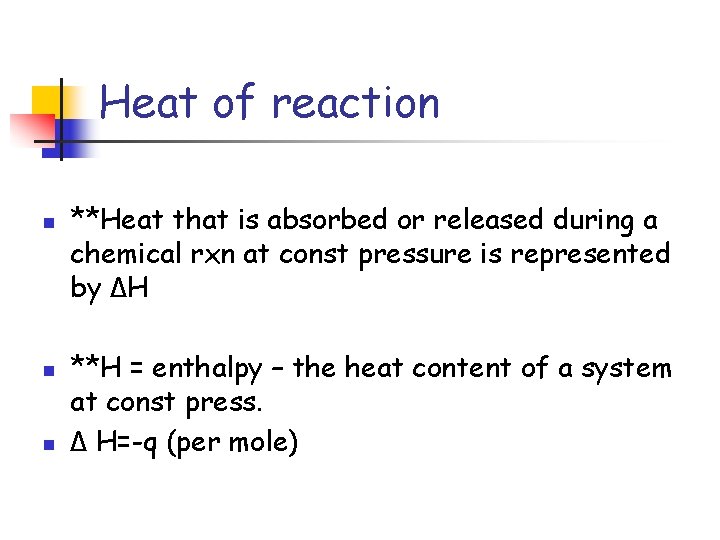
Heat of reaction n **Heat that is absorbed or released during a chemical rxn at const pressure is represented by ΔH **H = enthalpy – the heat content of a system at const press. Δ H=-q (per mole)

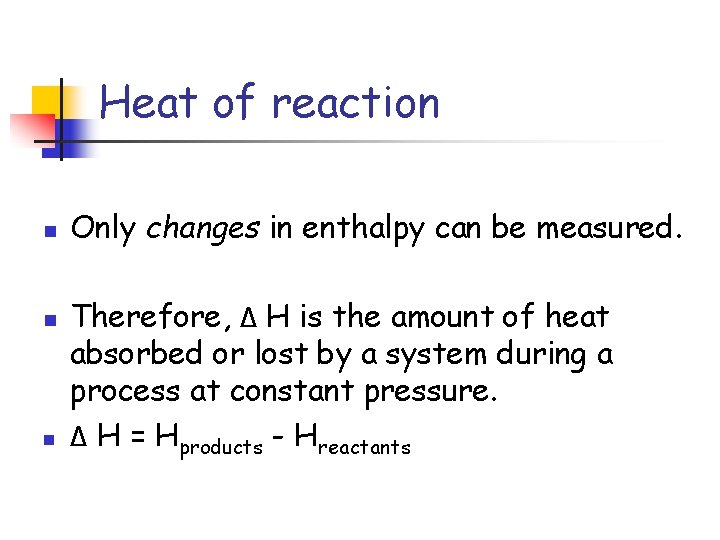
Heat of reaction n Only changes in enthalpy can be measured. Therefore, Δ H is the amount of heat absorbed or lost by a system during a process at constant pressure. Δ H = Hproducts - Hreactants

Heat of reaction n A thermometer can be used to follow heat flow during the reaction.

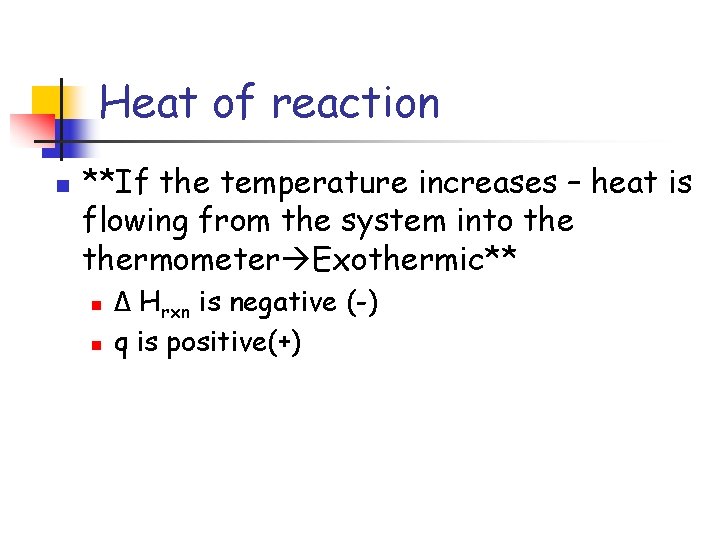
Heat of reaction n **If the temperature increases – heat is flowing from the system into thermometer Exothermic** n n Δ Hrxn is negative (-) q is positive(+)

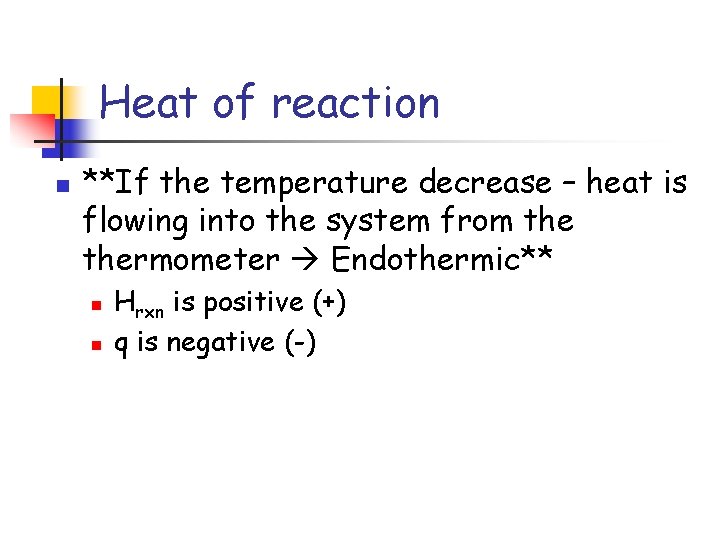
Heat of reaction n **If the temperature decrease – heat is flowing into the system from thermometer Endothermic** n n Hrxn is positive (+) q is negative (-)

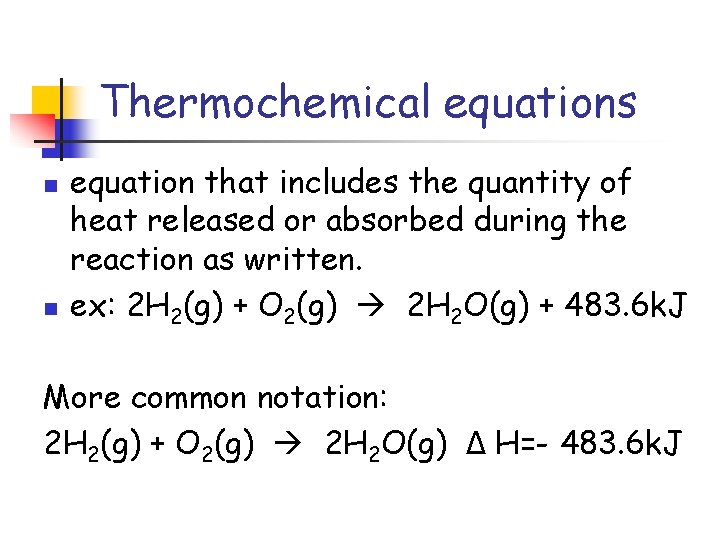
Thermochemical equations n n equation that includes the quantity of heat released or absorbed during the reaction as written. ex: 2 H 2(g) + O 2(g) 2 H 2 O(g) + 483. 6 k. J More common notation: 2 H 2(g) + O 2(g) 2 H 2 O(g) Δ H=- 483. 6 k. J

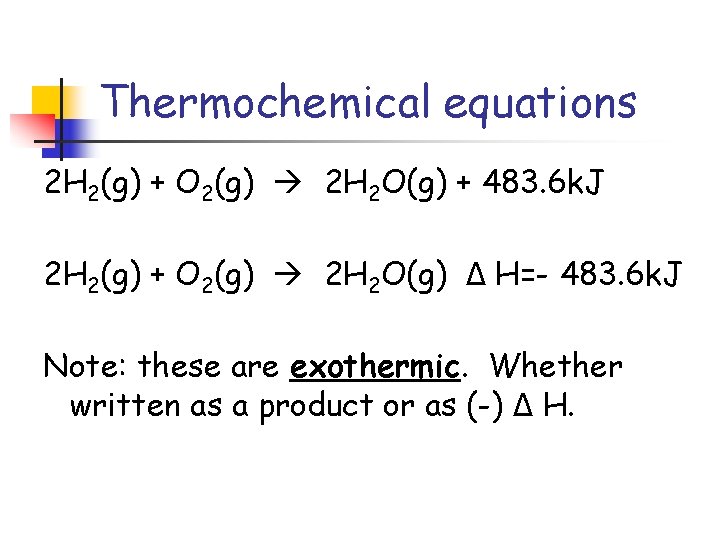
Thermochemical equations 2 H 2(g) + O 2(g) 2 H 2 O(g) + 483. 6 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) Δ H=- 483. 6 k. J Note: these are exothermic. Whether written as a product or as (-) Δ H.

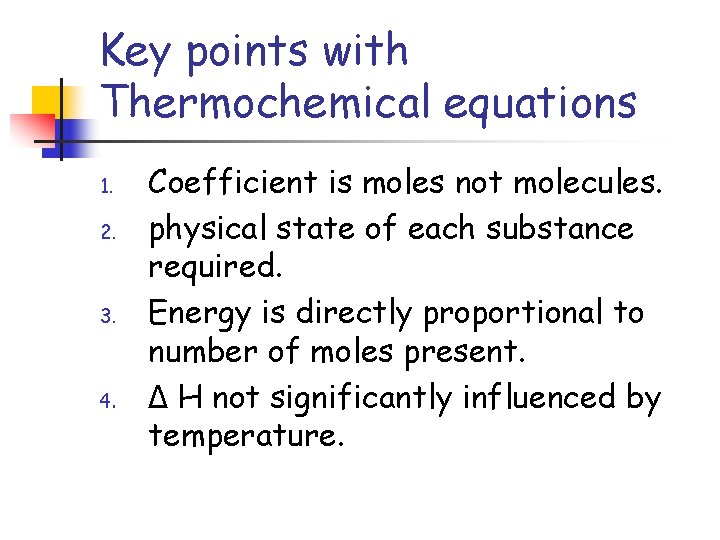
Key points with Thermochemical equations 1. 2. 3. 4. Coefficient is moles not molecules. physical state of each substance required. Energy is directly proportional to number of moles present. Δ H not significantly influenced by temperature.

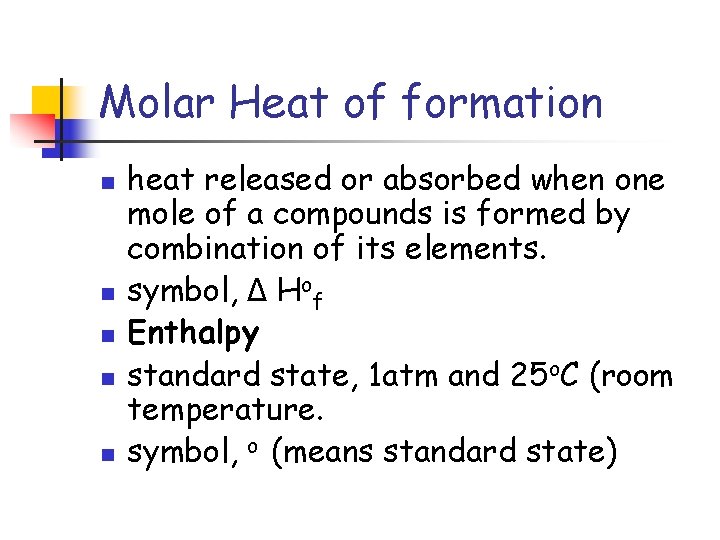
Molar Heat of formation n n heat released or absorbed when one mole of a compounds is formed by combination of its elements. symbol, Δ Hof Enthalpy standard state, 1 atm and 25 o. C (room temperature. symbol, o (means standard state)

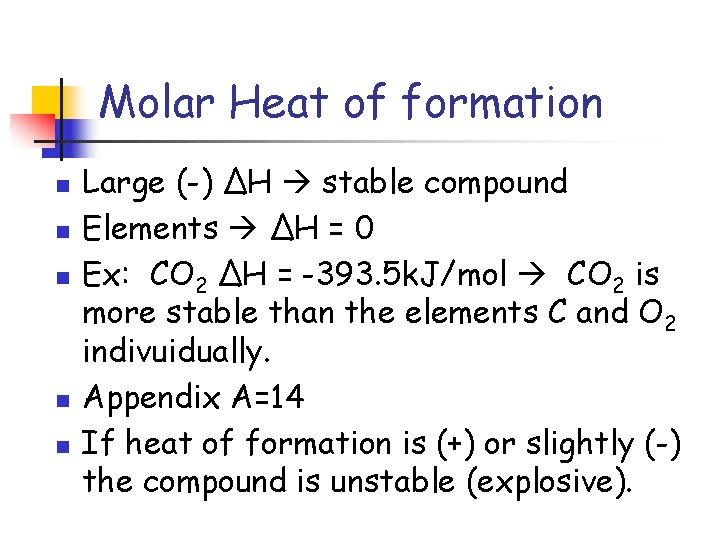
Molar Heat of formation n n Large (-) ΔH stable compound Elements ΔH = 0 Ex: CO 2 ΔH = -393. 5 k. J/mol CO 2 is more stable than the elements C and O 2 indivuidually. Appendix A=14 If heat of formation is (+) or slightly (-) the compound is unstable (explosive).

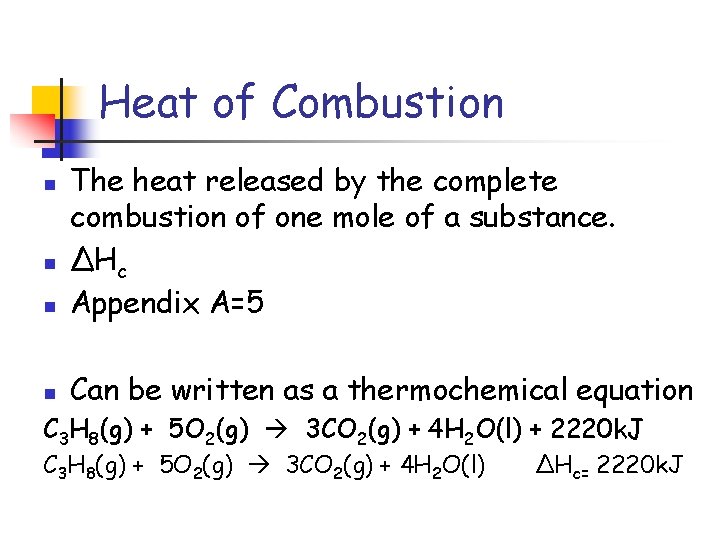
Heat of Combustion n The heat released by the complete combustion of one mole of a substance. ΔHc Appendix A=5 n Can be written as a thermochemical equation n n C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) + 2220 k. J C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) ΔHc= 2220 k. J

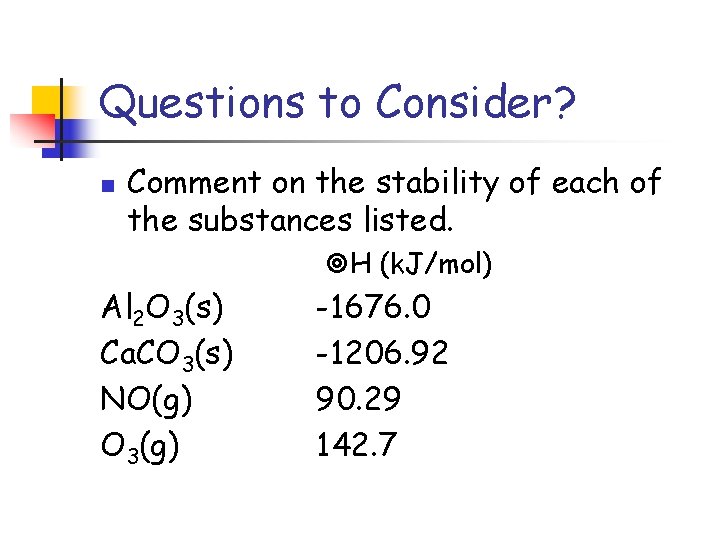
Questions to Consider? n Comment on the stability of each of the substances listed. H (k. J/mol) Al 2 O 3(s) Ca. CO 3(s) NO(g) O 3(g) -1676. 0 -1206. 92 90. 29 142. 7

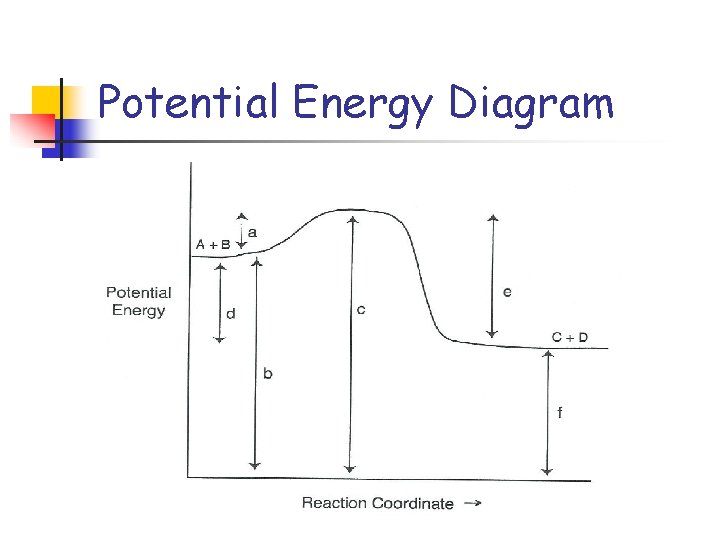
Potential Energy Diagram

Hess’s law n n The overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process. this is the formal definition that explains why Δ H = Hproducts - Hreactants

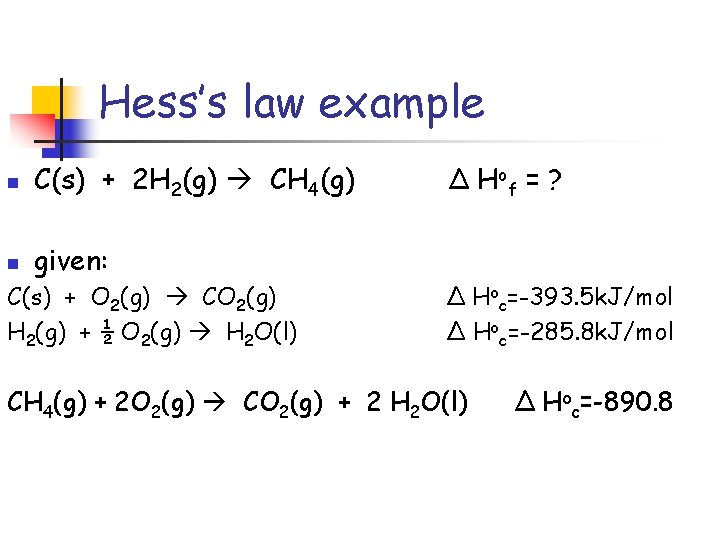
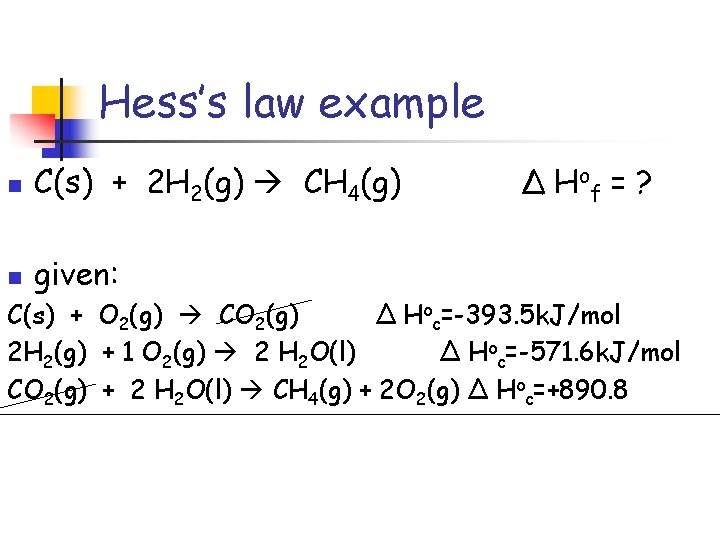
Hess’s law example n C(s) + 2 H 2(g) CH 4(g) n given: C(s) + O 2(g) CO 2(g) H 2(g) + ½ O 2(g) H 2 O(l) Δ H of = ? Δ Hoc=-393. 5 k. J/mol Δ Hoc=-285. 8 k. J/mol CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) Δ Hoc=-890. 8

Hess’s law problem rules 1. 2. If a reaction needs to be reversed, the sign of Δ Ho= is also reversed. Multiply the coefficients of the known equation so that when added together they give the desired thermochemcial equation.

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) Δ H of = ? Notice: methane is on the “wrong” side so it needs to be reversed. CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8 n

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) Δ H of = ? n Notice: hydrogen needs a coefficient of 2 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=2(-285. 8 k. J/mol)

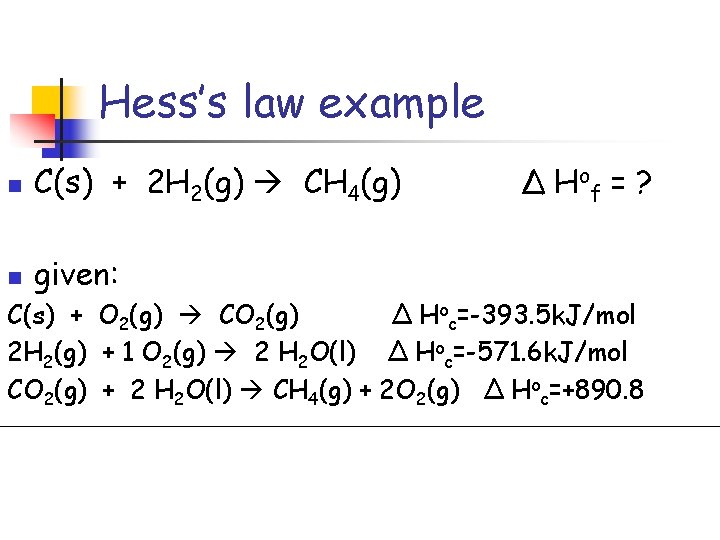
Hess’s law example n C(s) + 2 H 2(g) CH 4(g) n given: Δ H of = ? C(s) + O 2(g) CO 2(g) Δ Hoc=-393. 5 k. J/mol 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=-571. 6 k. J/mol CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) n given: Δ H of = ? C(s) + O 2(g) CO 2(g) Δ Hoc=-393. 5 k. J/mol 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=-571. 6 k. J/mol CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) n given: Δ H of = ? C(s) + O 2(g) CO 2(g) Δ Hoc=-393. 5 k. J/mol 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=-571. 6 k. J/mol CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) n given: Δ H of = ? C(s) + O 2(g) CO 2(g) Δ Hoc=-393. 5 k. J/mol 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=-571. 6 k. J/mol CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8

Hess’s law example n C(s) + 2 H 2(g) CH 4(g) Δ H of = ? given: C(s) + O 2(g) CO 2(g) Δ Hoc=-393. 5 k. J/mol 2 H 2(g) + 1 O 2(g) 2 H 2 O(l) Δ Hoc=-571. 6 k. J/mol CO 2(g) + 2 H 2 O(l) CH 4(g) + 2 O 2(g) Δ Hoc=+890. 8 C(s) + 2 H 2(g) CH 4(g) Δ Hof= -74. 3 k. J/mol n

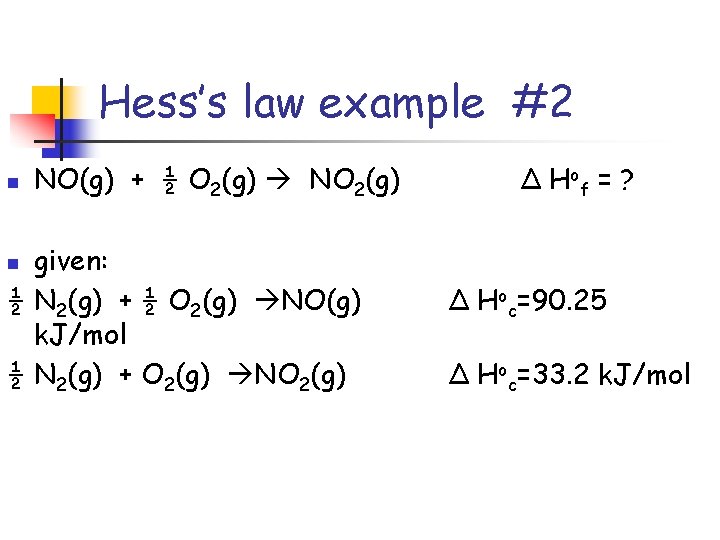
Hess’s law example #2 n NO(g) + ½ O 2(g) NO 2(g) given: ½ N 2(g) + ½ O 2(g) NO(g) k. J/mol ½ N 2(g) + O 2(g) NO 2(g) Δ H of = ? n Δ Hoc=90. 25 Δ Hoc=33. 2 k. J/mol
 Elizabeth mulroney
Elizabeth mulroney Examples for chemical change
Examples for chemical change Thermochemistry is study of
Thermochemistry is study of Kimia adalah
Kimia adalah Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry
Thermochemistry Thermochemistry is the study of *
Thermochemistry is the study of * Study of energy and its transformations
Study of energy and its transformations Which is an example of a physical change
Which is an example of a physical change A researcher decides to study cognitive changes
A researcher decides to study cognitive changes Kinetic energy thermochemistry
Kinetic energy thermochemistry Specific heat chem worksheet 16-1
Specific heat chem worksheet 16-1 Energy changes
Energy changes General chemistry thermochemistry
General chemistry thermochemistry Thermochemistry exam review
Thermochemistry exam review Thermochemistry equations
Thermochemistry equations Introduction to thermochemistry
Introduction to thermochemistry Thermochemistry video
Thermochemistry video Balanced thermochemical equation
Balanced thermochemical equation Q=mc∆t
Q=mc∆t Thermochemistry cartoon
Thermochemistry cartoon Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry
Thermochemistry What is thermochemistry
What is thermochemistry Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Hess law constant heat summation
Hess law constant heat summation Internal thermal energy
Internal thermal energy Thermochemistry conversions
Thermochemistry conversions Energy diagram thermochemistry
Energy diagram thermochemistry Ap chemistry thermodynamics
Ap chemistry thermodynamics Chapter 6 thermochemistry
Chapter 6 thermochemistry Thermochemistry form 3 exercise
Thermochemistry form 3 exercise Thermochemistry equations
Thermochemistry equations Thermochemistry review
Thermochemistry review Gibbs free energy unit
Gibbs free energy unit Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Now answer these questions
Now answer these questions Thermochemistry equations
Thermochemistry equations Thermochemistry is concerned with the
Thermochemistry is concerned with the Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Unit 7 ap chemistry
Unit 7 ap chemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemistry one pager
Thermochemistry one pager Thermochemistry
Thermochemistry Bomb calorimetry equation
Bomb calorimetry equation Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Thermochemistry concepts
Thermochemistry concepts Thermochemistry equations
Thermochemistry equations How to calculate the heat absorbed
How to calculate the heat absorbed Cartoon thermochemistry
Cartoon thermochemistry Difference between time study and motion study
Difference between time study and motion study Marty lobdell study less study smart
Marty lobdell study less study smart Case series
Case series Phytogeographical regions of world
Phytogeographical regions of world Retrospective cohort study vs prospective cohort study
Retrospective cohort study vs prospective cohort study Time study objectives
Time study objectives Work study technique
Work study technique Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết V. c c
V. c c Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi điện thế nghỉ
điện thế nghỉ Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là