Chapters 16 17 Thermochemistry Thermochemistry The study of









![Equilibrium � Rate of forward reaction = rate of reverse reaction � [products] and Equilibrium � Rate of forward reaction = rate of reverse reaction � [products] and](https://slidetodoc.com/presentation_image_h2/40dd22ab1f185ff66471cf4214ef6a39/image-33.jpg)
- Slides: 33

Chapters 16 & 17 Thermochemistry

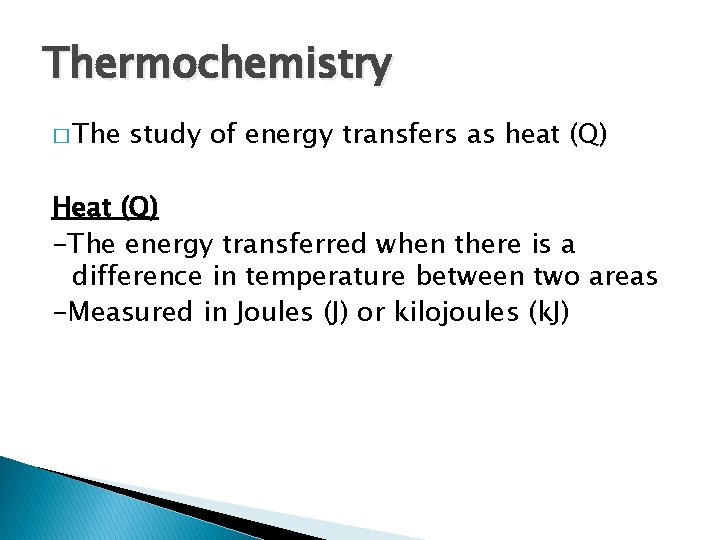
Thermochemistry � The study of energy transfers as heat (Q) Heat (Q) -The energy transferred when there is a difference in temperature between two areas -Measured in Joules (J) or kilojoules (k. J)

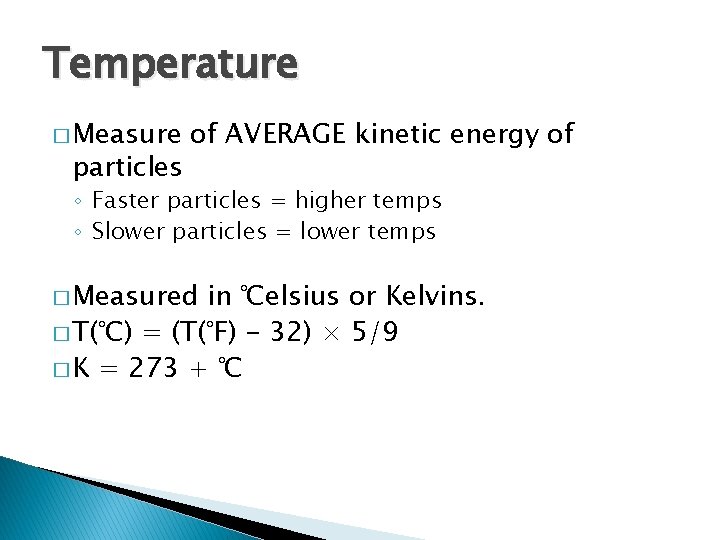
Temperature � Measure particles of AVERAGE kinetic energy of ◦ Faster particles = higher temps ◦ Slower particles = lower temps � Measured in ℃elsius or Kelvins. � T(°C) = (T(°F) - 32) × 5/9 � K = 273 + ℃

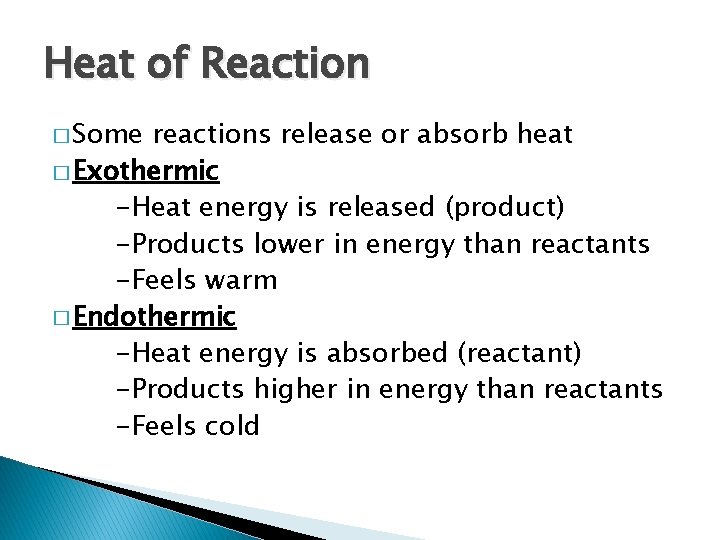
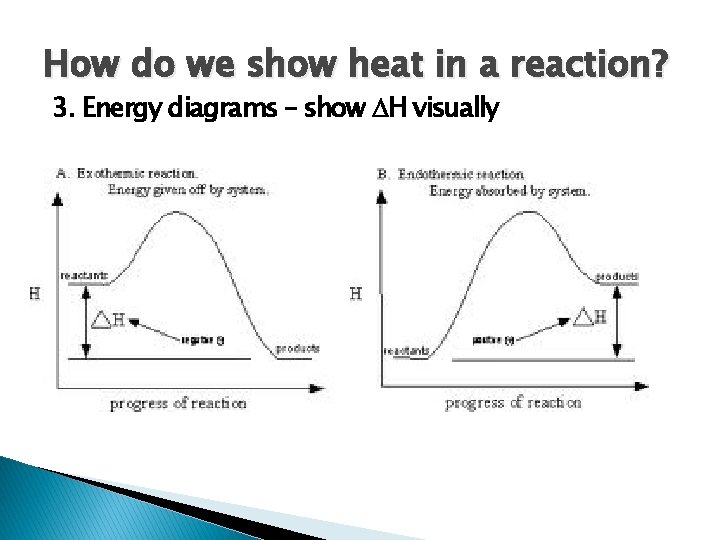
Heat of Reaction � Some reactions release or absorb heat � Exothermic -Heat energy is released (product) -Products lower in energy than reactants -Feels warm � Endothermic -Heat energy is absorbed (reactant) -Products higher in energy than reactants -Feels cold

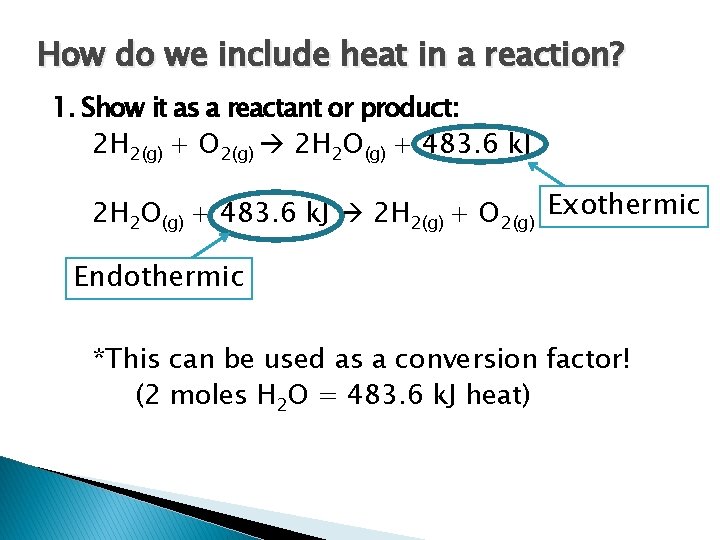
How do we include heat in a reaction? 1. Show it as a reactant or product: 2 H 2(g) + O 2(g) 2 H 2 O(g) + 483. 6 k. J 2 H 2(g) + O 2(g) Exothermic Endothermic *This can be used as a conversion factor! (2 moles H 2 O = 483. 6 k. J heat)

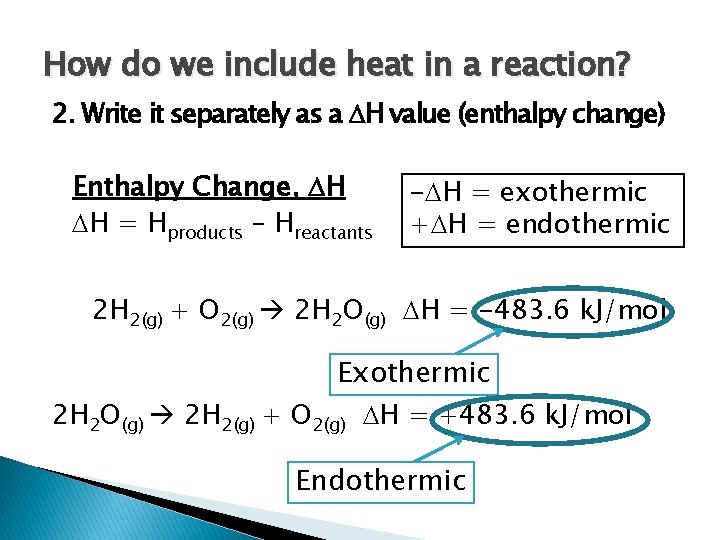
How do we include heat in a reaction? 2. Write it separately as a H value (enthalpy change) Enthalpy Change, H H = Hproducts – Hreactants - H = exothermic + H = endothermic 2 H 2(g) + O 2(g) 2 H 2 O(g) H = -483. 6 k. J/mol Exothermic 2 H 2 O(g) 2 H 2(g) + O 2(g) H = +483. 6 k. J/mol Endothermic

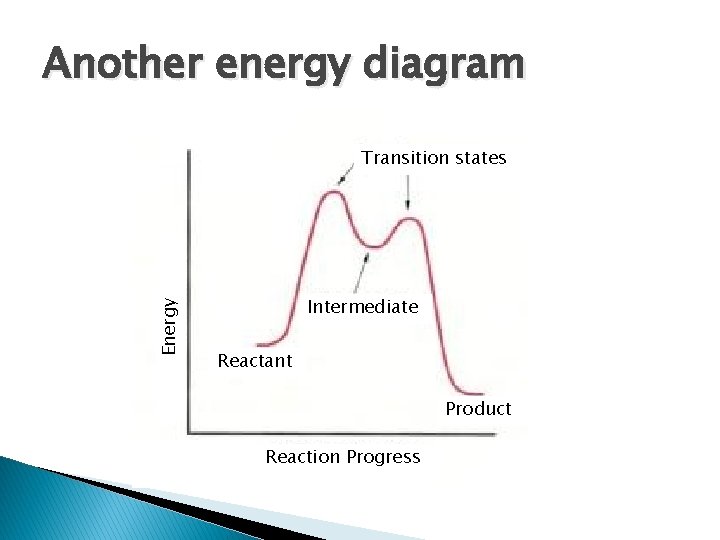
How do we show heat in a reaction? 3. Energy diagrams – show H visually

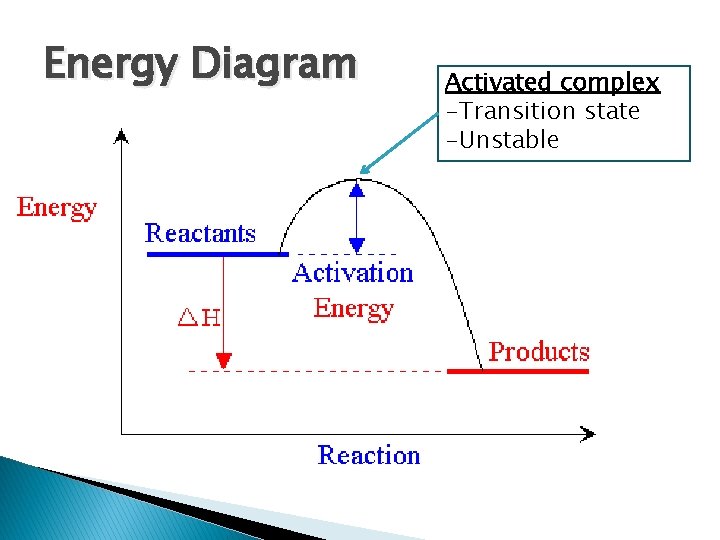
Energy Diagram Activated complex -Transition state -Unstable

Another energy diagram Energy Transition states Intermediate Reactant Product Reaction Progress

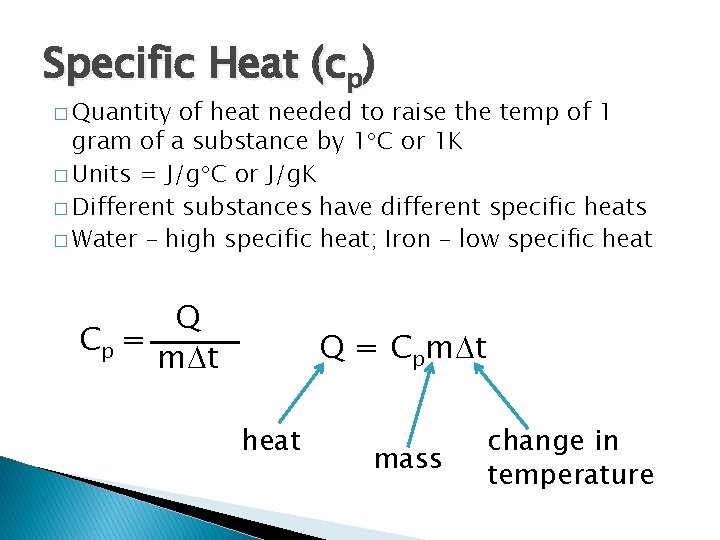
Specific Heat (cp) � Quantity of heat needed to raise the temp of 1 gram of a substance by 1 C or 1 K � Units = J/g C or J/g. K � Different substances have different specific heats � Water – high specific heat; Iron – low specific heat Q Cp = m t Q = Cpm t heat mass change in temperature

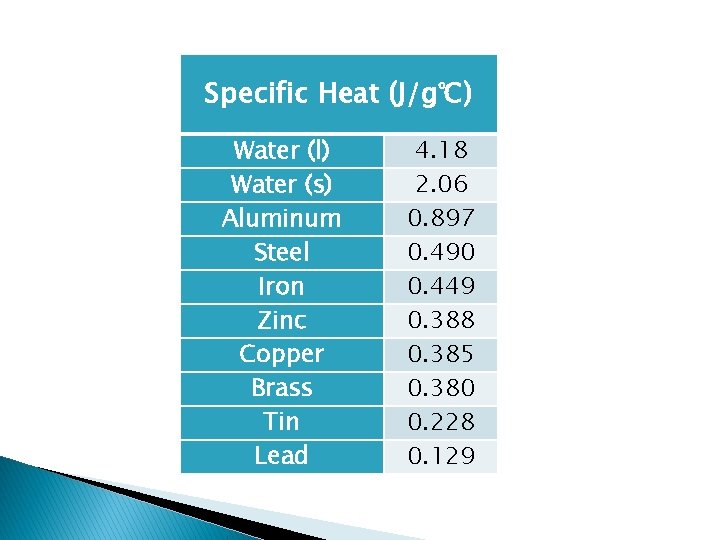
Specific Heat (J/g℃) Water (l) Water (s) Aluminum Steel Iron Zinc Copper Brass Tin Lead 4. 18 2. 06 0. 897 0. 490 0. 449 0. 388 0. 385 0. 380 0. 228 0. 129


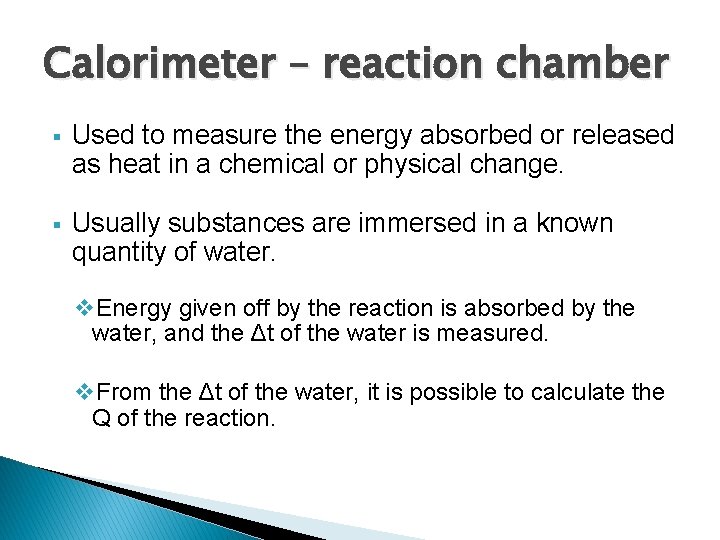
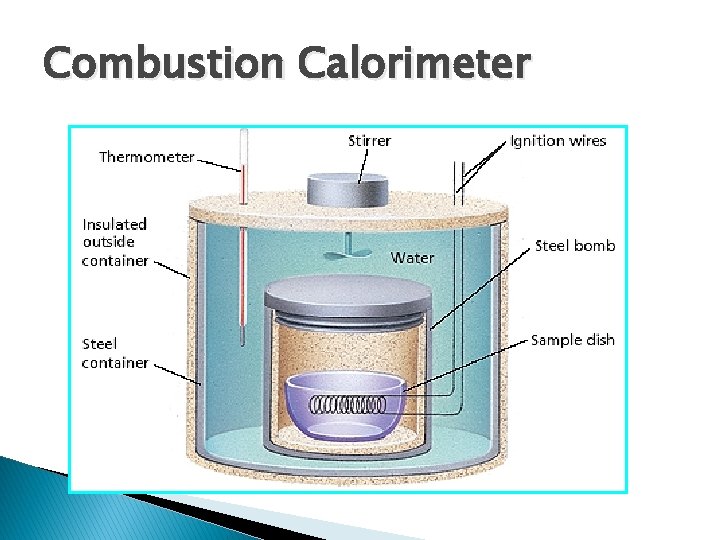
Calorimeter – reaction chamber § Used to measure the energy absorbed or released as heat in a chemical or physical change. § Usually substances are immersed in a known quantity of water. v. Energy given off by the reaction is absorbed by the water, and the Δt of the water is measured. v. From the Δt of the water, it is possible to calculate the Q of the reaction.

Combustion Calorimeter

Examples: pg. ) 534 � Determine the specific heat of a material if a 35 g sample absorbed 96 J as it was heated from 293 K to 313 K.

� If 980 k. J of energy are added to 6. 2 L of water at 291 K, what will the final temperature of the water be?

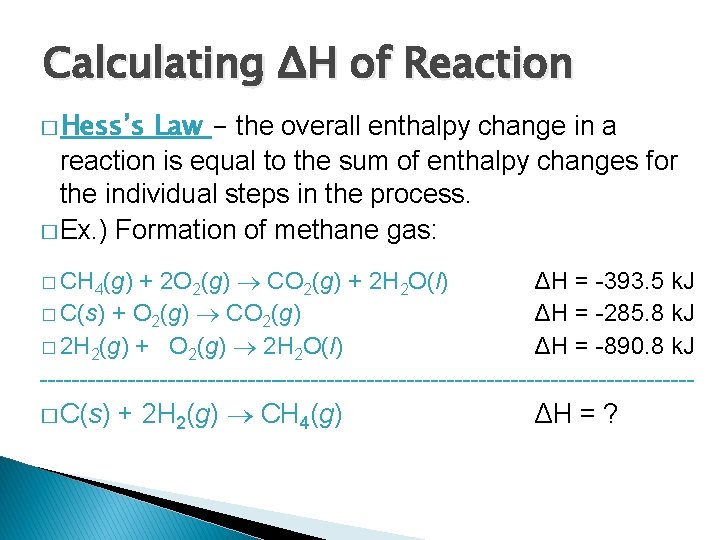
Calculating ΔH of Reaction � Hess’s Law - the overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process. � Ex. ) Formation of methane gas: + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔH = -393. 5 k. J � C(s) + O 2(g) CO 2(g) ΔH = -285. 8 k. J � 2 H 2(g) + O 2(g) 2 H 2 O(l) ΔH = -890. 8 k. J -----------------------------------------� CH 4(g) � C(s) + 2 H 2(g) CH 4(g) ΔH = ?

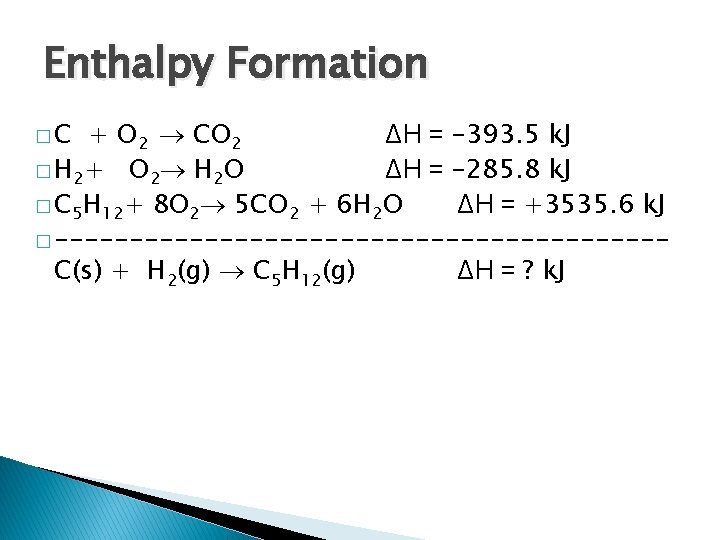
Enthalpy Formation �C + O 2 CO 2 ΔH = -393. 5 k. J � H 2+ O 2 H 2 O ΔH = -285. 8 k. J � C 5 H 12+ 8 O 2 5 CO 2 + 6 H 2 O ΔH = +3535. 6 k. J � --------------------C(s) + H 2(g) C 5 H 12(g) ΔH = ? k. J

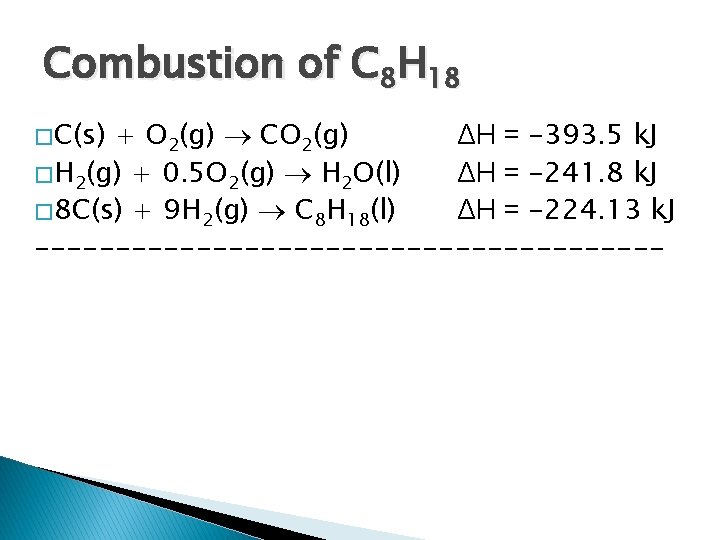
Combustion of C 8 H 18 � C(s) + O 2(g) CO 2(g) ΔH = -393. 5 k. J � H 2(g) + 0. 5 O 2(g) H 2 O(l) ΔH = -241. 8 k. J � 8 C(s) + 9 H 2(g) C 8 H 18(l) ΔH = -224. 13 k. J --------------------

Driving Forces of Reactions � Not all reactions are spontaneous � Spontaneity depends on 1. Enthalpy ( H) 2. Entropy ( S)

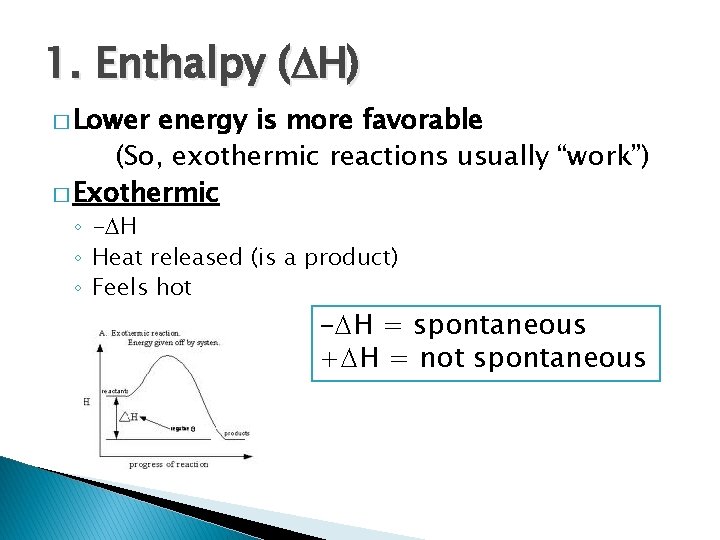
1. Enthalpy ( H) � Lower energy is more favorable (So, exothermic reactions usually “work”) � Exothermic ◦ - H ◦ Heat released (is a product) ◦ Feels hot - H = spontaneous + H = not spontaneous

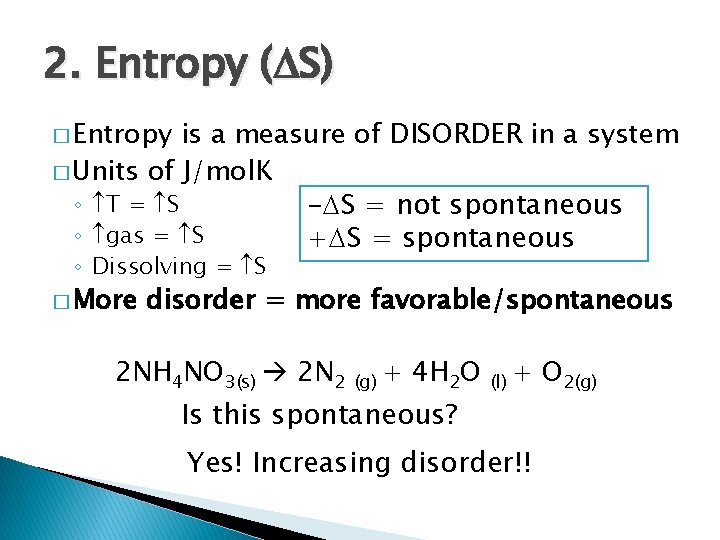
2. Entropy ( S) � Entropy is a measure of DISORDER in a system � Units of J/mol. K ◦ T = S - S = not spontaneous ◦ gas = S + S = spontaneous ◦ Dissolving = S � More disorder = more favorable/spontaneous 2 NH 4 NO 3(s) 2 N 2 (g) + 4 H 2 O Is this spontaneous? (l) + O 2(g) Yes! Increasing disorder!!

What if H and S compete? � Put them together!! � Use Gibb’s Free Energy calculation

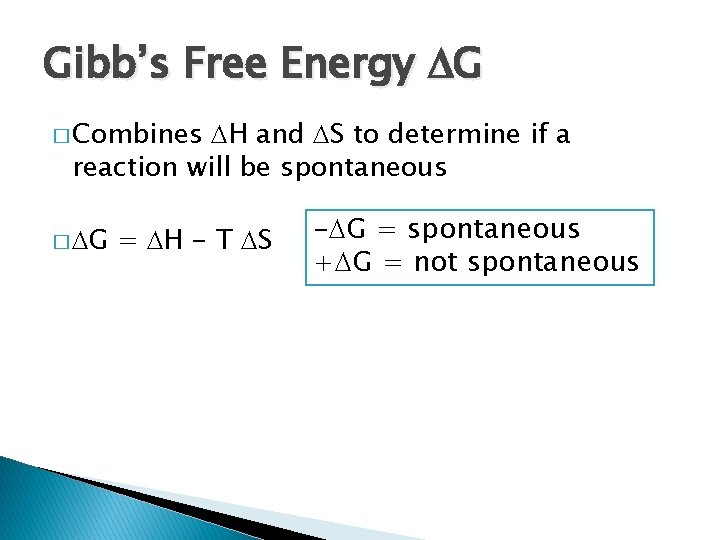
Gibb’s Free Energy G � Combines H and S to determine if a reaction will be spontaneous � G = H - T S - G = spontaneous + G = not spontaneous

Collision Theory � Collision Theory ◦ Particles must collide with enough energy and force at a proper orientation in order for a reaction to occur � Activation energy, Ea : - Energy required for a reaction to occur


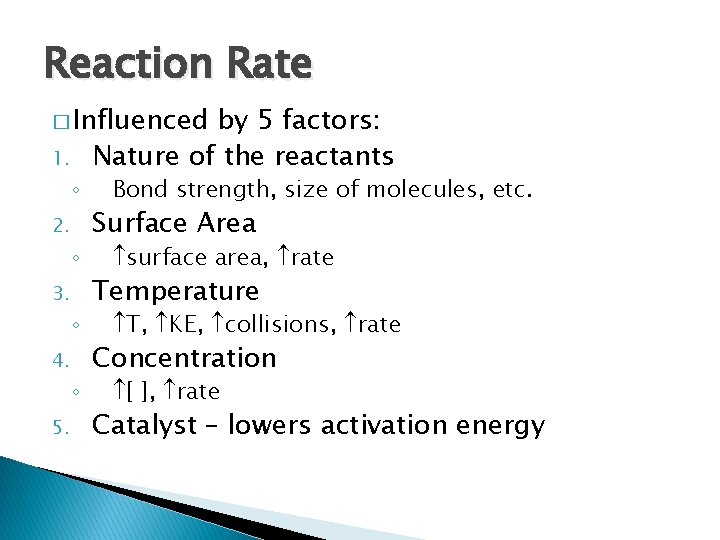
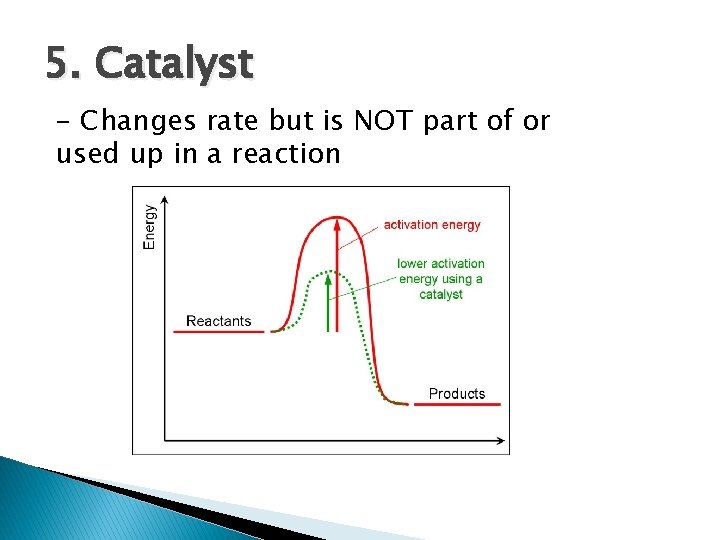
Reaction Rate � Influenced 1. ◦ 2. ◦ 3. ◦ 4. ◦ 5. by 5 factors: Nature of the reactants Bond strength, size of molecules, etc. Surface Area surface area, rate Temperature T, KE, collisions, rate Concentration [ ], rate Catalyst – lowers activation energy

5. Catalyst - Changes rate but is NOT part of or used up in a reaction

Chapter Review �Pg 552 �#’s 1, 3 -4, 7 -10, 12, 23 -25 �Due day of test

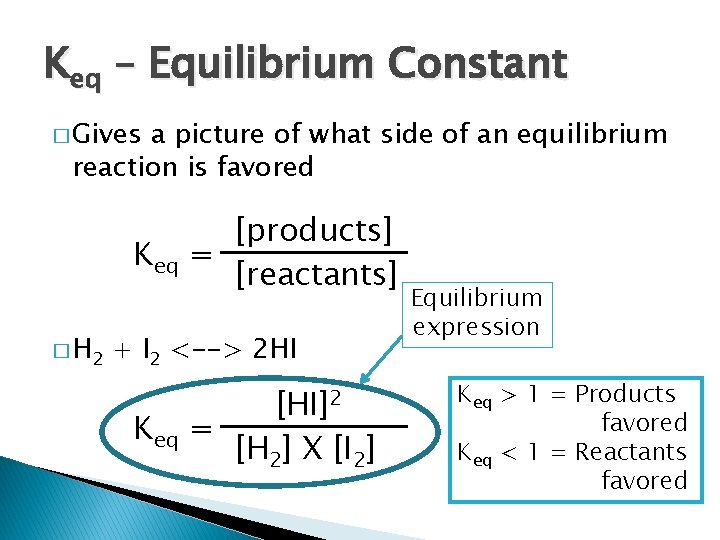
Keq – Equilibrium Constant � Gives a picture of what side of an equilibrium reaction is favored Keq � H 2 [products] = [reactants] + I 2 <--> 2 HI Keq [HI]2 = [H 2] X [I 2] Equilibrium expression Keq > 1 = Products favored Keq < 1 = Reactants favored

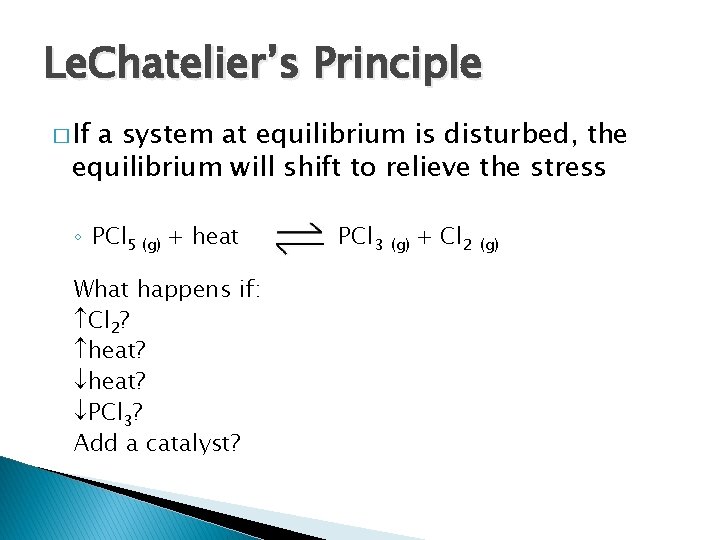
Le. Chatelier’s Principle � If a system at equilibrium is disturbed, the equilibrium will shift to relieve the stress ◦ PCl 5 (g) + heat What happens if: Cl 2? heat? PCl 3? Add a catalyst? PCl 3 (g) + Cl 2 (g)

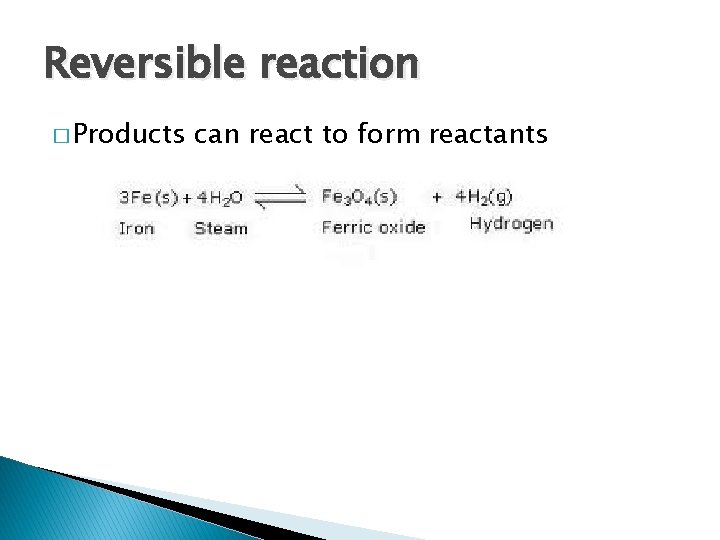
Reversible reaction � Products can react to form reactants
![Equilibrium Rate of forward reaction rate of reverse reaction products and Equilibrium � Rate of forward reaction = rate of reverse reaction � [products] and](https://slidetodoc.com/presentation_image_h2/40dd22ab1f185ff66471cf4214ef6a39/image-33.jpg)
Equilibrium � Rate of forward reaction = rate of reverse reaction � [products] and [reactants] is unchanged � Is dynamic (always changing) � Sometimes favors one side: HBr + H 2 O H 3 O+ + Br. H 2 CO 3 + H 2 O H 3 O+ + HCO 3 -
 Thermochemistry is the study of *
Thermochemistry is the study of * Study of energy transformations
Study of energy transformations Thermochemistry is study of
Thermochemistry is study of Termokimia adalah cabang ilmu kimia yang mempelajari
Termokimia adalah cabang ilmu kimia yang mempelajari Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of
Thermochemistry is study of Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry
Thermochemistry The hunger games chapter 4
The hunger games chapter 4 Chapter questions for to kill a mockingbird part 1
Chapter questions for to kill a mockingbird part 1 Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là V cc cc
V cc cc Slidetodoc
Slidetodoc Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Lời thề hippocrates
Lời thề hippocrates đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ