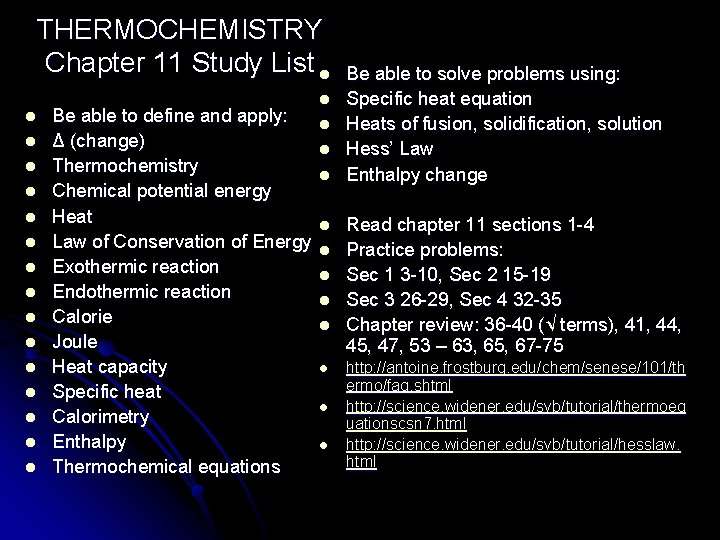
THERMOCHEMISTRY Chapter 11 THERMOCHEMISTRY Chapter 11 Study List





















- Slides: 40

THERMOCHEMISTRY Chapter 11

THERMOCHEMISTRY Chapter 11 Study List l l l l Be able to define and apply: Δ (change) Thermochemistry Chemical potential energy Heat Law of Conservation of Energy Exothermic reaction Endothermic reaction Calorie Joule Heat capacity Specific heat Calorimetry Enthalpy Thermochemical equations l l l Be able to solve problems using: Specific heat equation Heats of fusion, solidification, solution Hess’ Law Enthalpy change Read chapter 11 sections 1 -4 Practice problems: Sec 1 3 -10, Sec 2 15 -19 Sec 3 26 -29, Sec 4 32 -35 Chapter review: 36 -40 (√ terms), 41, 44, 45, 47, 53 – 63, 65, 67 -75 http: //antoine. frostburg. edu/chem/senese/101/th ermo/faq. shtml http: //science. widener. edu/svb/tutorial/thermoeq uationscsn 7. html http: //science. widener. edu/svb/tutorial/hesslaw. html

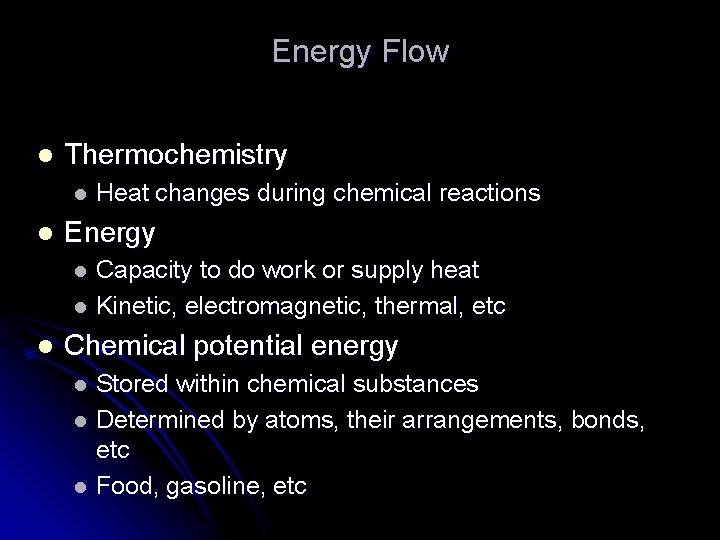
Energy Flow l Thermochemistry l l Heat changes during chemical reactions Energy Capacity to do work or supply heat l Kinetic, electromagnetic, thermal, etc l l Chemical potential energy Stored within chemical substances l Determined by atoms, their arrangements, bonds, etc l Food, gasoline, etc l

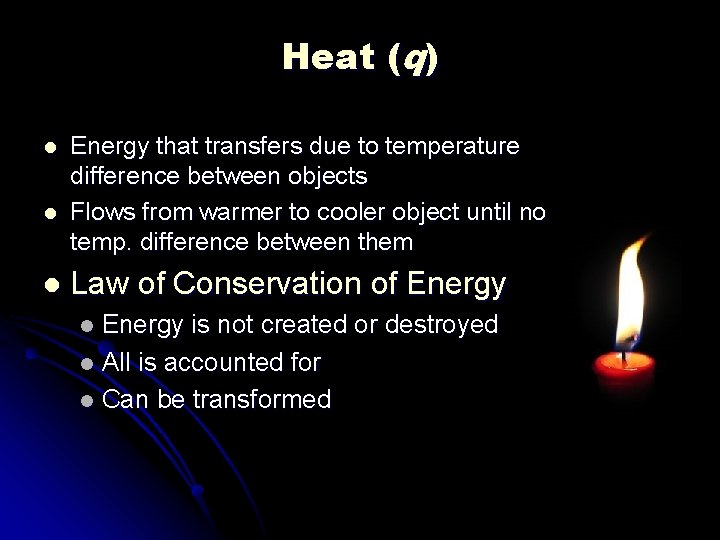
Heat (q) l l l Energy that transfers due to temperature difference between objects Flows from warmer to cooler object until no temp. difference between them Law of Conservation of Energy is not created or destroyed l All is accounted for l Can be transformed l

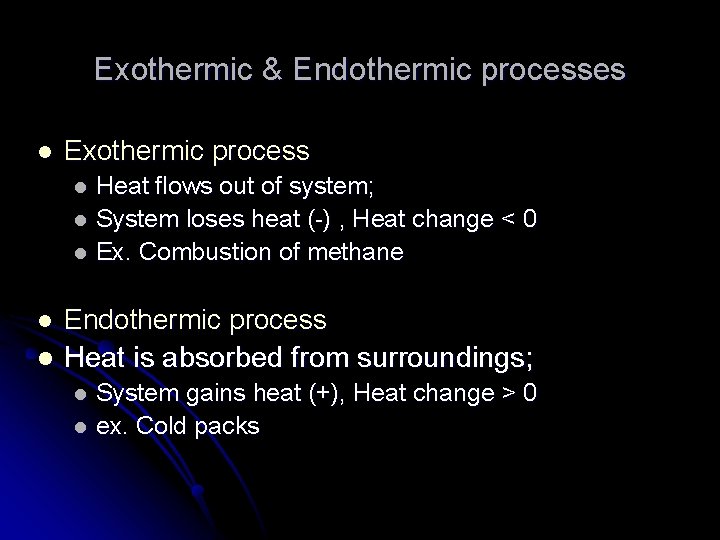
Exothermic & Endothermic processes l Exothermic process Heat flows out of system; l System loses heat (-) , Heat change < 0 l Ex. Combustion of methane l l l Endothermic process Heat is absorbed from surroundings; System gains heat (+), Heat change > 0 l ex. Cold packs l

Units of heat l calorie (cal) l Amount of heat needed to raise the temperature of 1 g of water 1 o C l 1000 calories = 1 kilocalorie= 1 kcal l (heat cal is not to be confused with dietary calories, = 1000 heat calories) l Joule (J) l Metric unit of heat & energy l 1 J = 0. 2390 cal 1 cal = 4. 184 J

Convert these: l It takes 50. 2 J to raise the temp of a 100. 0 g piece of glass. How many calories? l 50. 2 l J x 0. 239 cal = 12. 0 cal 1 J A small chocolate bar has about 210, 000 cal. How many Joules? l 210, 000 l cal x 4. 184 J = 878, 640 J or 1 cal 880 k. J (sig fig)

Specific Heat l Heat capacity l l Amount of heat needed to increase the temp of the object 1 o C Specific heat Amount of heat needed to raise the temp of 1 g of substance 1 o C l C = ___q_____ C= specific heat q = heat l m x ΔT m = mass ΔT = change in temp l

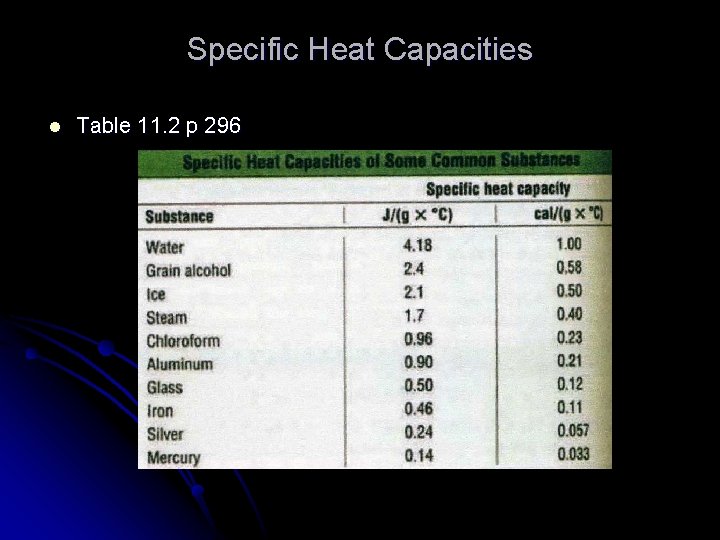
Specific Heat Capacities l Table 11. 2 p 296

Calculating specific heat l l A 1. 55 g piece of stainless steel absorbs 141 J of heat when its temperature increases by 178 o. C. What is the specific heat of stainless steel? l Know: m= 1. 55 g, q=141 J, ΔT = 178 o. C. l C = ___q_____ m x ΔT l C = 141 J = 1. 55 g x 178 o. C 0. 511 J/go. C

And more… l How much heat is required to raise the temperature of 250. 0 g of mercury 52 o. C? C = ___q_____ q=m. CΔT m x ΔT l CHg = 0. 14 J/go. C (table 11. 2) l q=250. 0 g x 0. 14 J/go. C x 52 o. C l 1820 J or 1. 8 k. J l

Give these a try l 435 J of heat is added to 3. 4 g of olive oil at 21 o. C, the temperature increases to 85 o. C. What is the specific heat of olive oil? C= 435 J_____ l 3. 4 g x (85 o. C -21 o. C) l 2. 0 J/go. C l l How much heat is required to raise the temperature of 400. 0 g of silver 45 o. C? q=m. CΔT CAg= 0. 24 J/go. C l q =400. 0 g x 0. 24 J/go. C x 45 o. C l 4320 J or 4. 3 k. J l

Calorimetry & Thermochemical Equations l Calorimetry Precise measurement of heat change in physical or chemical processes l Heat released by the system must equal the heat absorbed by the surroundings. Why? l l Calorimeter Instrument used to measure amount of heat liberated or absorbed during a change l Constant l Pressure calorimeter l

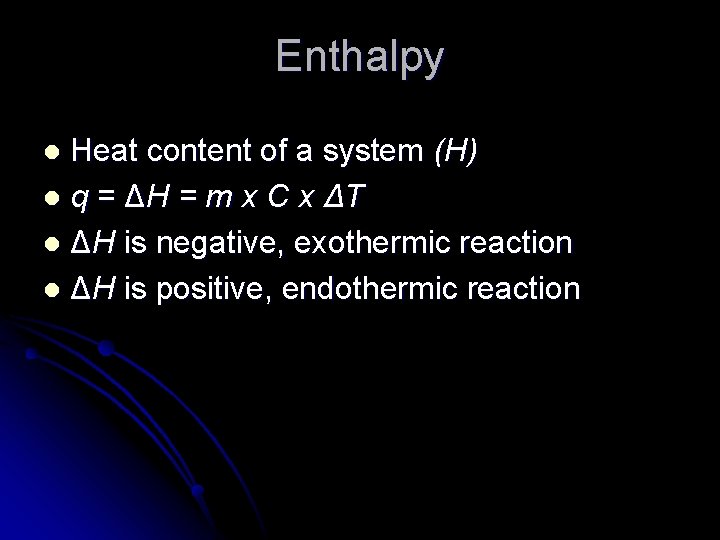
Enthalpy Heat content of a system (H) l q = ΔH = m x C x ΔT l ΔH is negative, exothermic reaction l ΔH is positive, endothermic reaction l

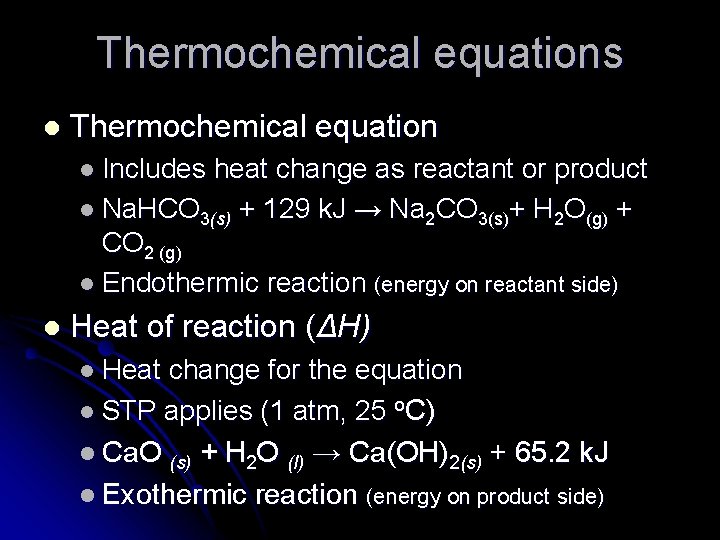
Thermochemical equations l Thermochemical equation l Includes heat change as reactant or product l Na. HCO 3(s) + 129 k. J → Na 2 CO 3(s)+ H 2 O(g) + CO 2 (g) l Endothermic reaction (energy on reactant side) l Heat of reaction (ΔH) l Heat change for the equation l STP applies (1 atm, 25 o. C) l Ca. O (s) + H 2 O (l) → Ca(OH)2(s) + 65. 2 k. J l Exothermic reaction (energy on product side)

Solving enthalpy problems l l Amount of heat absorbed or released depends on: number of moles of substance l l physical state of reactants and products-solid, liquid, gas l l (stoichiometry returns!) Each has different heat of combustion Heat of reaction for complete burning Mol Z x __ΔH___ = heat released/absorbed in reaction #mol Z in equation

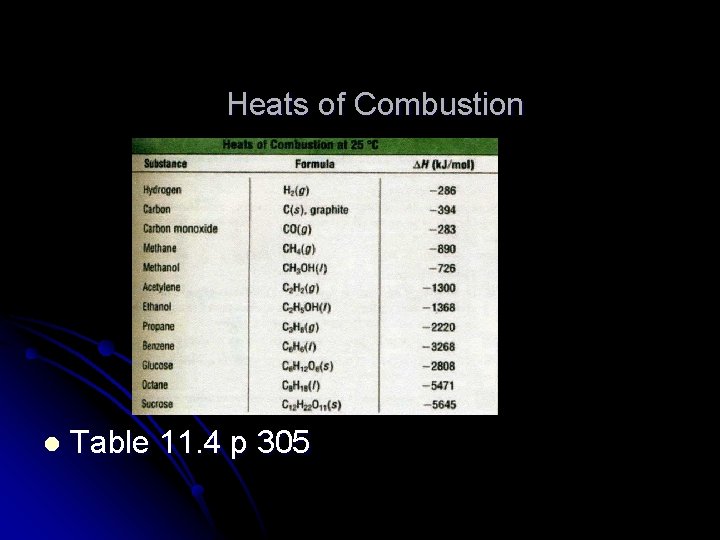
Heats of Combustion l Table 11. 4 p 305

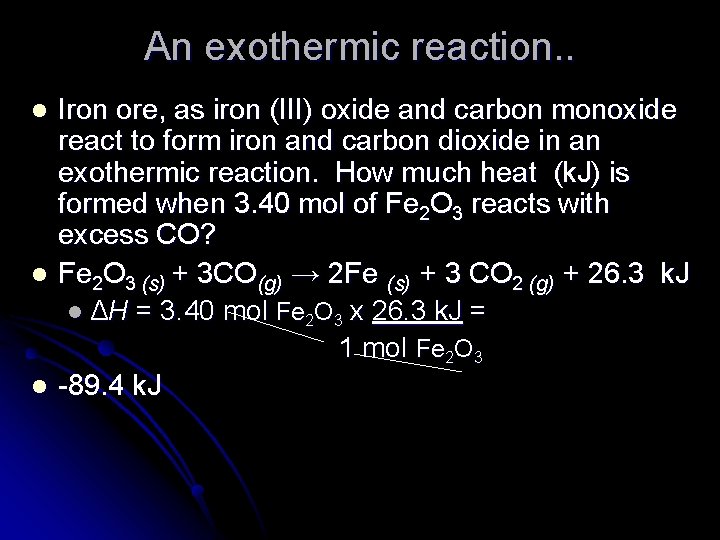
An exothermic reaction. . l l l Iron ore, as iron (III) oxide and carbon monoxide react to form iron and carbon dioxide in an exothermic reaction. How much heat (k. J) is formed when 3. 40 mol of Fe 2 O 3 reacts with excess CO? Fe 2 O 3 (s) + 3 CO(g) → 2 Fe (s) + 3 CO 2 (g) + 26. 3 k. J l ΔH = 3. 40 mol Fe 2 O 3 x 26. 3 k. J = 1 mol Fe 2 O 3 -89. 4 k. J

l l When carbon disulfide forms from its elements, heat is absorbed. Calculate the amount of heat (J) absorbed when 5. 66 g of carbon disulfide is formed. C(s) + 2 S(s) + 89. 3 k. J→CS 2 (l) l 5. 66 g CS 2 x 1 mol =0. 0744 mol 76. 1 g CS 2 l 0. 0744 mol CS 2 x 89. 3 k. J = 1 mol CS 2 l 6. 64 k. J

Try this one l l How many kilojoules of heat are released when 0. 75 mol of Mg burn with excess O 2? 2 Mg(s) + O 2(g) → 2 Mg. O(g) + 1204 k. J l l 0. 75 mol Mg x 1204 k. J 2 mol Mg =-451. 5 or 450 k. J

Heat and changes of state l Phase changes involve adding or losing heat energy (Ch. 10) l Temperature remains constant until phase change complete l same amount of energy @ same temp Solid ↔ liquid; liquid ↔ gas l

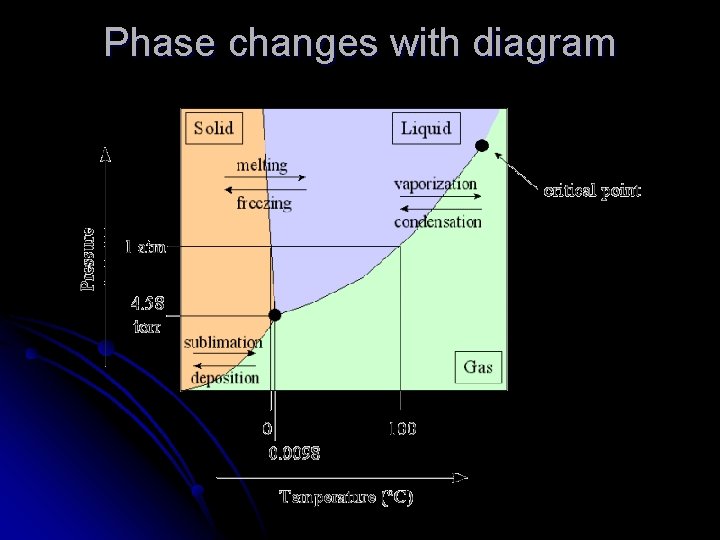
Phase changes l l l Phase diagram Shows the relationship between pressure, temperature and phase of substance Triple point-temp and pressure conditions where substance can exist in all three phases

Phase changes l l Substances change phase as energy is added to or lost from substance Particles slow down (energy loss) or speed up (energy gain) Energy gain-melting, vaporization/boiling, sublimation Energy loss-freezing, condensation, deposition

And those words are. . l l Sublimation-solid to gas without passing through liquid phase l High vapor pressure l Examples- CO 2, ice in freezer, iodine Deposition-gas to solid without passing through liquid phase l Examples-Snow, frost

Phase changes with diagram

At the freezing point… l Molar heat of fusion l l Molar heat of solidification l l Amount of energy absorbed by one mole of substance as melts from solid to liquid Amount of energy lost by one mole of substance as it solidifies from a liquid ΔHfus= - ΔHsolid ΔHfus water is 6. 01 k. J/mol l ΔHsolid water is -6. 01 k. J/mol l

At the boiling point l Molar heat of vaporization l l Molar heat of condensation l l Amount of energy absorbed by one mole of substance as vaporizes from liquid to gas Amount of energy lost by one mole of substance as it changes gas to liquid ΔHvap= - ΔHcond ΔHvap water is 40. 7 k. J/mol l ΔHcond water is -40. 7 k. J/mol l

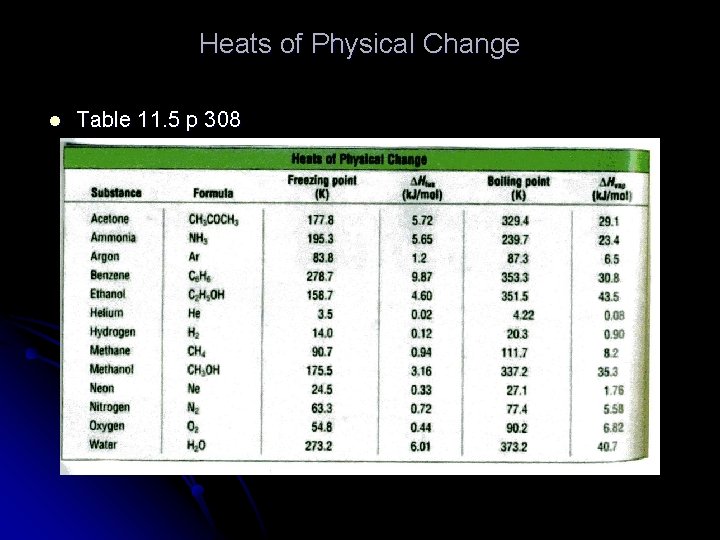
Heats of Physical Change l Table 11. 5 p 308

l l At 0 o. C and 1 atm. , how many grams of ice could be melted by adding 5. 0 k. J of heat? ΔHfus water is 6. 01 k. J/mol, ΔH= 5. 0 k. J l l 5. 0 k. J x 1 mol ice = 0. 832 mol 6. 01 k. J 0. 832 mol x 18. 0 g = 14. 976 or 15 g of ice 1 mol

Heats of solution l l Changes in heat can occur when solute dissolves in solvent Molar heat of solution -heat change caused by dissolving 1 mol of substance ΔHsoln positive - endothermic reaction O NH + NH 4 NO 3 (s)H→ + NO ΔHsoln = 25. 7 4 (aq) 3 (aq) k. J/mol l 2 l ΔHsoln negative-exothermic reaction Ca. Cl 2(s) → Ca 2+ (aq) + 2 Cl- (aq) k. J/mol ΔHsoln = - 82. 8

Try this one. . l How many kilojoules of heat is released when 25. 0 g of Na. OH is dissolved in water? ΔHsoln=-445. 1 k. J/mol l 25. 0 g x 1 mol x -445. 1 k. J = -278 k. J 40. 0 g 1 mol l How many mol of NH 4 NO 3(s) must be dissolved in water so that 88. 0 k. J of heat is released by the water? ΔHsoln = -25. 7 k. J/mol l l -88. 0 k. J x 1 mol = 3. 42 k. J -25. 7 k. J Time for section 3 problems, p. 313

Calculating Heat Changes l Hess’s law of heat summation If a reaction occurs in several different steps and the heats of reaction of each are known, l then the heat of reaction for the total can be calculated by summing the ΔH of the steps l l Ex. carbon undergoes combustion to form carbon monoxide in two steps: l C + O 2 →CO 2 (g) ΔH = -394 k. J l CO 2 (g) → CO + ½ O 2(g) ΔH = 283 k. J l - 394 k. J + 283 k. J = -111 k. J

Find ΔH for the formation of water vapor from liquid water l l l Is it endo- or exothermic? H 2 (g) + ½ O 2(g) → H 2 O (g) Δ H 0 f = -57. 8 kcal H 2(g) + ½ O 2(g) → H 2 O (l) Δ H 0 f = -68. 3 kcal Since changing from liquid to gas, reverse second equation H 2 O (l) → H 2(g) + ½ O 2(g) Δ H 0 f = 68. 3 kcal l And that gives H 2 O (l) + H 2(g) + ½ O 2(g) → H 2 (g) + ½ O 2(g) + H 2 O (g) l l l X off “spectators” to give H 2 O (l) → H 2 O (g) 68. 3 kcal + -57. 8 kcal = 10. 5 kcal

Standard Heat (Enthalpy) of Formation l l Change in enthalpy that occurs with the formation of 1 mol of this substance from its elements Occurs at standard state- 1 atm @ 25 o C. Element is in physical state that occurs at standard state- i. e. O 2(g). Δ H 0 f (products) - Δ H 0 f (reactants) = Δ H 0

l Δ H 0 f of free elements set at 0 l l l include diatomics such as H 2, O 2, N 2, Cl 2, l 2 etc Enthalpies with positive value endothermic Enthalpies with negative value exothermic

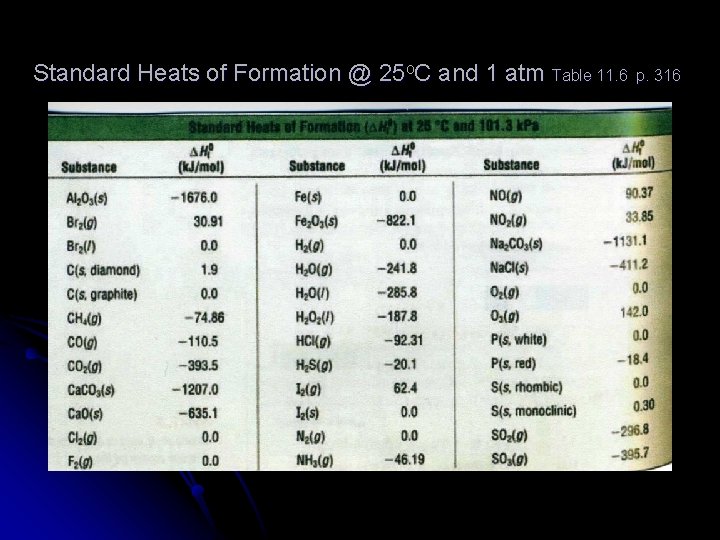
Standard Heats of Formation @ 25 o. C and 1 atm Table 11. 6 p. 316

Calculate Δ H 0 Br 2(g) → Br 2(l) l Δ H 0 f (products) - Δ H 0 f (reactants) = Δ H 0 Br 2(g) = 30. 91 k. J/mol Δ H 0 Br 2(g) = 0. 0 k. J/mol l 30. 91 k. J/mol - 0. 0 k. J/mol = 30. 91 k. J/mol l l

Calculate Δ H 0 for 2 SO 2(g) + O 2(g) → 2 SO 3(g) l Δ H 0 f reactants: l l Δ H 0 f products: l l (2 mol SO 2(g) x -296. 8 k. J ) + (1 mol O 2(g) x 0. 0 k. J) = -593. 6 k. J 1 mol SO 2(g) 1 mol O 2(g) 2 mol SO 3(g) X -395. 7 k. J = -791. 4 k. J 1 mol SO 3(g) Δ H 0 f (products) - Δ H 0 f (reactants): -791. 4 k. J - -593. 6 k. J = - 197. 8 k. J l Standard heat values are from table 11. 6 l

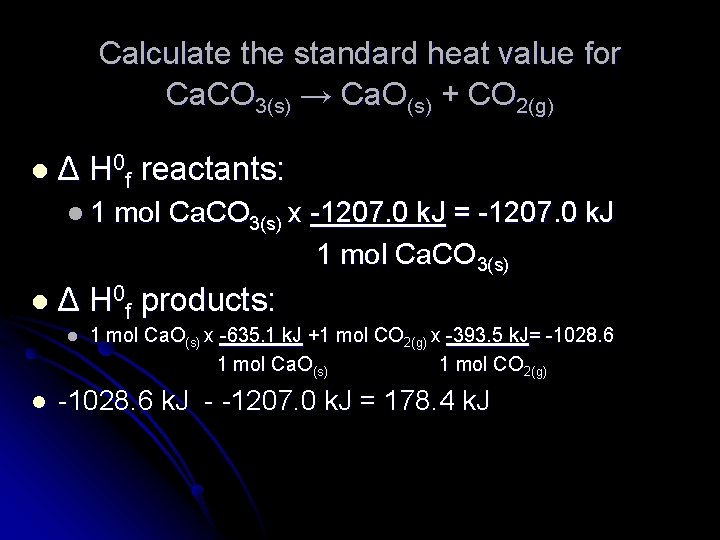
Calculate the standard heat value for Ca. CO 3(s) → Ca. O(s) + CO 2(g) lΔ H 0 f reactants: l 1 lΔ l l mol Ca. CO 3(s) x -1207. 0 k. J = -1207. 0 k. J 1 mol Ca. CO 3(s) H 0 f products: 1 mol Ca. O(s) x -635. 1 k. J +1 mol CO 2(g) x -393. 5 k. J= -1028. 6 1 mol Ca. O(s) 1 mol CO 2(g) -1028. 6 k. J - -1207. 0 k. J = 178. 4 k. J

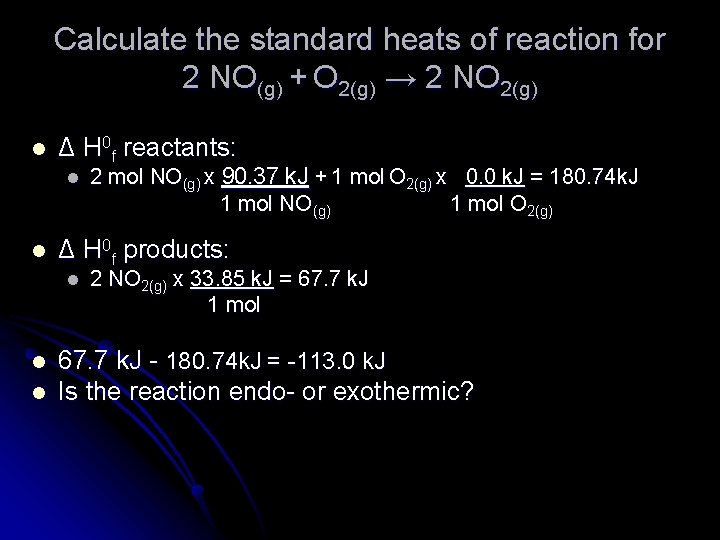
Calculate the standard heats of reaction for 2 NO(g) + O 2(g) → 2 NO 2(g) l Δ H 0 f reactants: l l Δ H 0 f products: l l l 2 mol NO(g) x 90. 37 k. J + 1 mol O 2(g) x 0. 0 k. J = 180. 74 k. J 1 mol NO(g) 1 mol O 2(g) 2 NO 2(g) x 33. 85 k. J = 67. 7 k. J 1 mol 67. 7 k. J - 180. 74 k. J = -113. 0 k. J Is the reaction endo- or exothermic?