Thermochemistry AP Chem Ch 6 Thermochemistry the study








- Slides: 20

Thermochemistry AP Chem Ch. 6

Thermochemistry – the study of heat changes that accompany chemical reactions and phase changes Universe = System + Surroundings l Endothermic Reaction – one in which energy (heat) is absorbed into the system l Exothermic Reaction – one in which energy (heat) is released from the system l

Energy l Energy – the ability to do work or produce heat l the SI unit for heat is the Joule (J) l Heat CANNOT be measured directly; it is calculated l “q” = quantity of heat

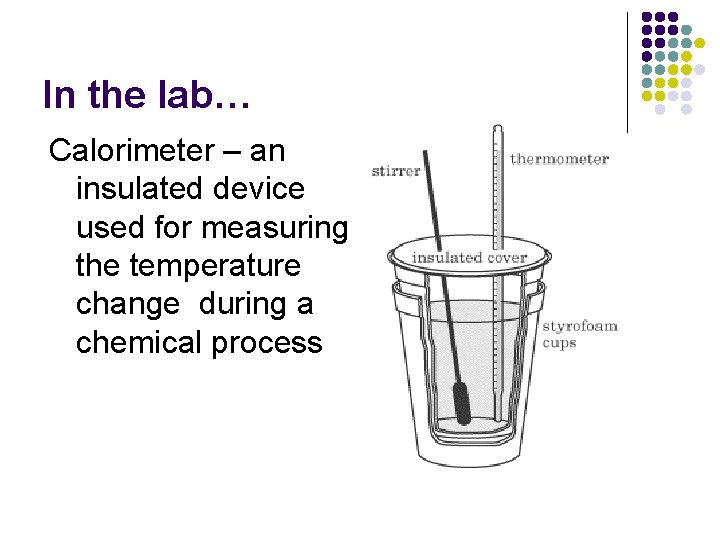
In the lab… Calorimeter – an insulated device used for measuring the temperature change during a chemical process

Useful Conversion Factors 1 cal = 4. 184 J 1 Cal = 1000 cal 101. 3 J = 1 L • atm 1 J = 1 kg • m 2 / s 2

Specific Heat l l Specific Heat – energy required to raise the temperature of one gram of any substance one degree Celsius. Each substance has its own specific heat l The higher the specific heat, the longer it will take to raise the temp. l water has a very high specific heat 4. 184 J/g°C

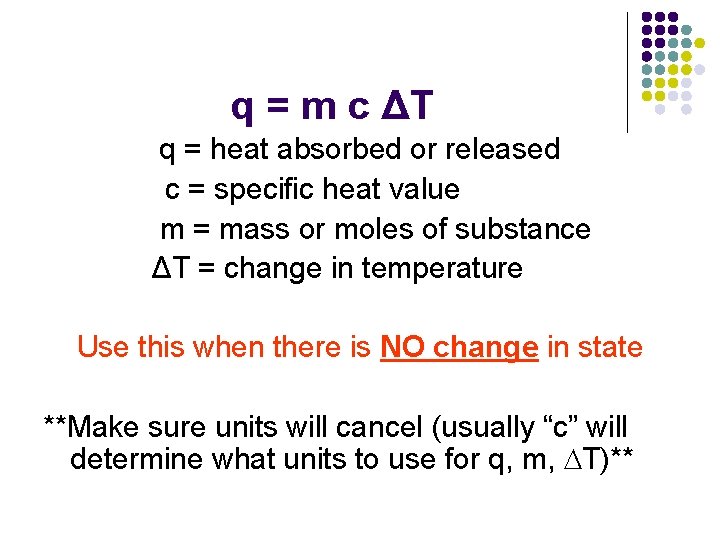
q = m c ΔT q = heat absorbed or released c = specific heat value m = mass or moles of substance ΔT = change in temperature Use this when there is NO change in state **Make sure units will cancel (usually “c” will determine what units to use for q, m, DT)**

Heat Lost = Heat Gained l When 2 substances at different temperatures come into contact with each other, heat is transferred from the warmer to the cooler substance until both substances are at the same temperature

Enthalpy l l Enthalpy (H) – measure of the amount of energy aborbed/released by a reaction Changes in enthalpy (ΔH rxn )can be calculated for specific chemical reactions Exothermic (lose heat) = -ΔHrxn Endothermic (gains heat) = +ΔHrxn

Enthalpy (cont’d) ΔH rxn = H products – H reactants l DH = q only at constant pressure l ΔH° = standard enthalpy change l l ° = occurs under standard conditions l standard conditions = 1 atm, 298 K (25°C)

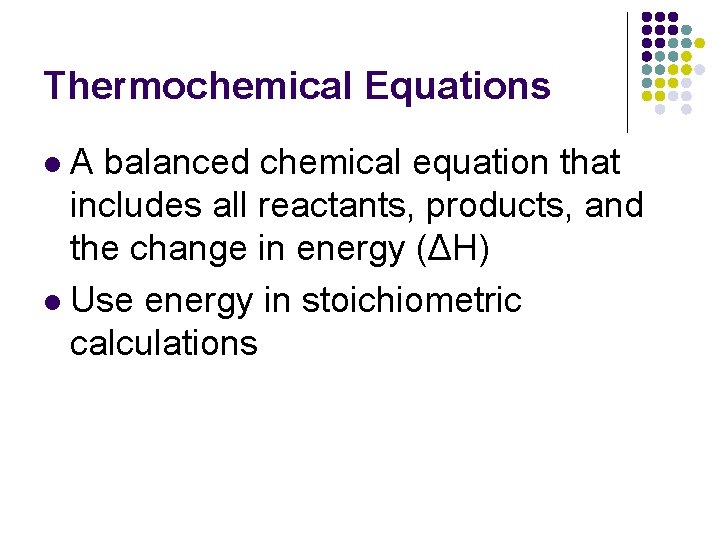
Thermochemical Equations A balanced chemical equation that includes all reactants, products, and the change in energy (ΔH) l Use energy in stoichiometric calculations l

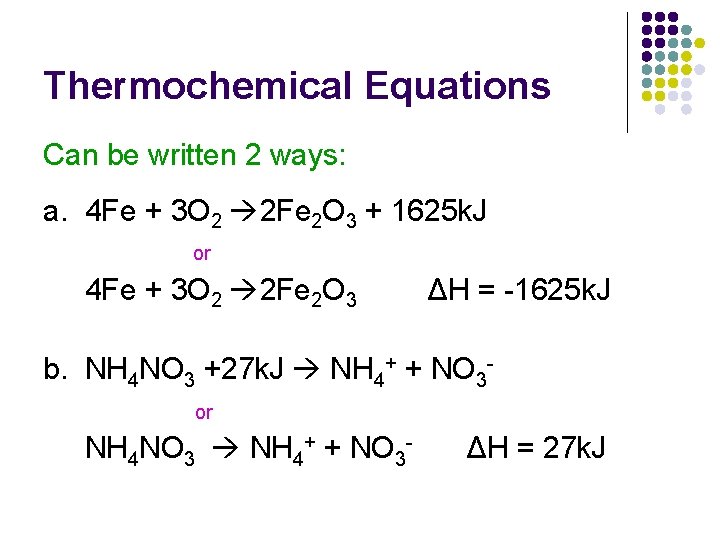
Thermochemical Equations Can be written 2 ways: a. 4 Fe + 3 O 2 2 Fe 2 O 3 + 1625 k. J or 4 Fe + 3 O 2 2 Fe 2 O 3 ΔH = -1625 k. J b. NH 4 NO 3 +27 k. J NH 4+ + NO 3 or NH 4 NO 3 NH 4+ + NO 3 - ΔH = 27 k. J

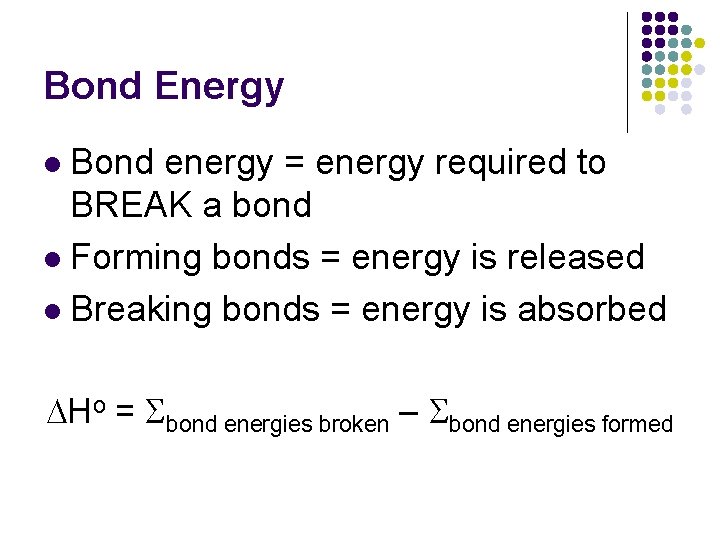
Bond Energy Bond energy = energy required to BREAK a bond l Forming bonds = energy is released l Breaking bonds = energy is absorbed l DHo = bond energies broken – bond energies formed

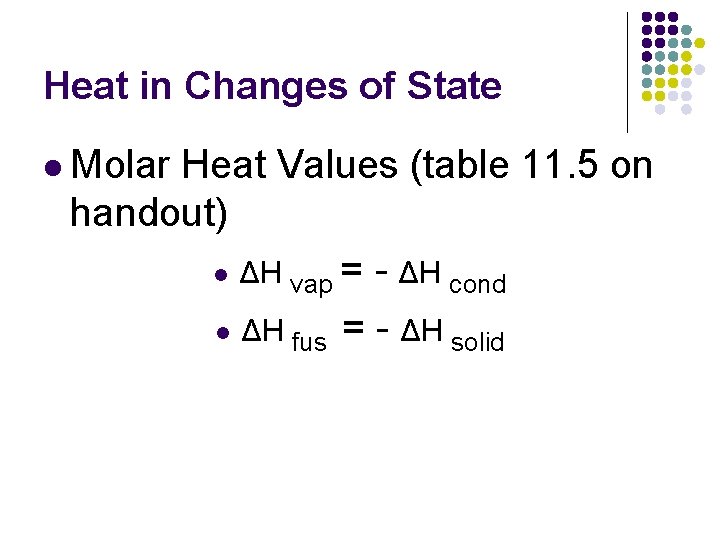
Heat in Changes of State l Molar Heat Values (table 11. 5 on handout) l ΔH vap = - ΔH cond l ΔH fus = - ΔH solid

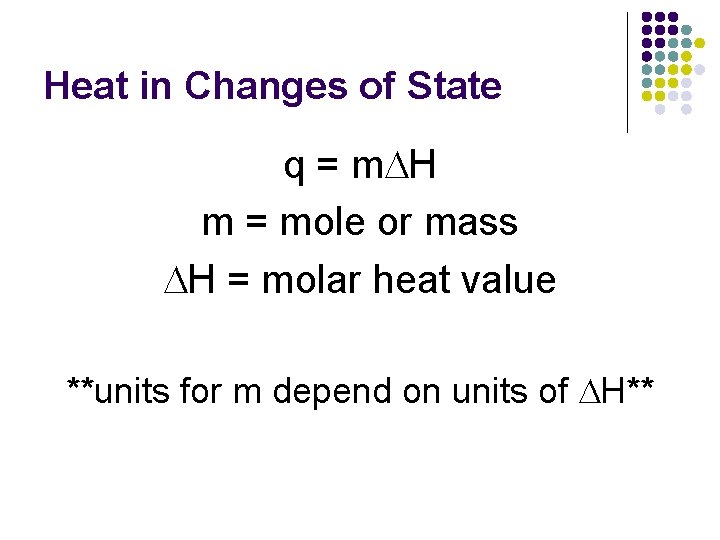
Heat in Changes of State q = m. DH m = mole or mass DH = molar heat value **units for m depend on units of DH**

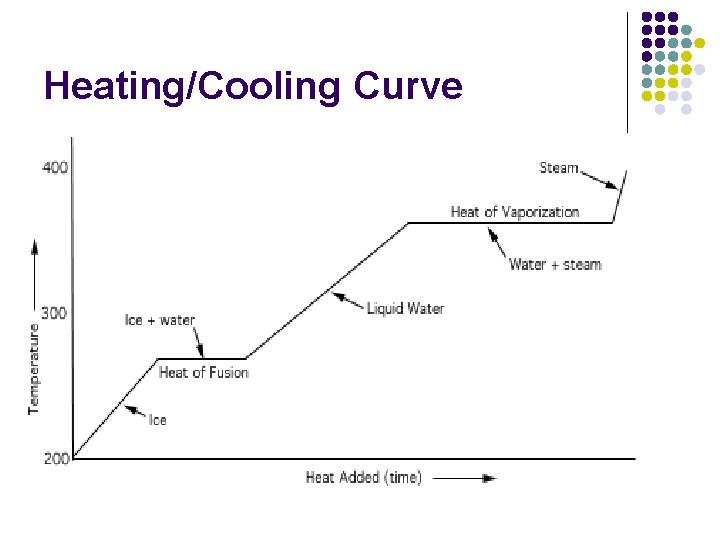
Heating/Cooling Curve

Changes in Temp & State Use Heating/Cooling Curve l Use q=mc. Dt and q=m. DH to calculate total heat l

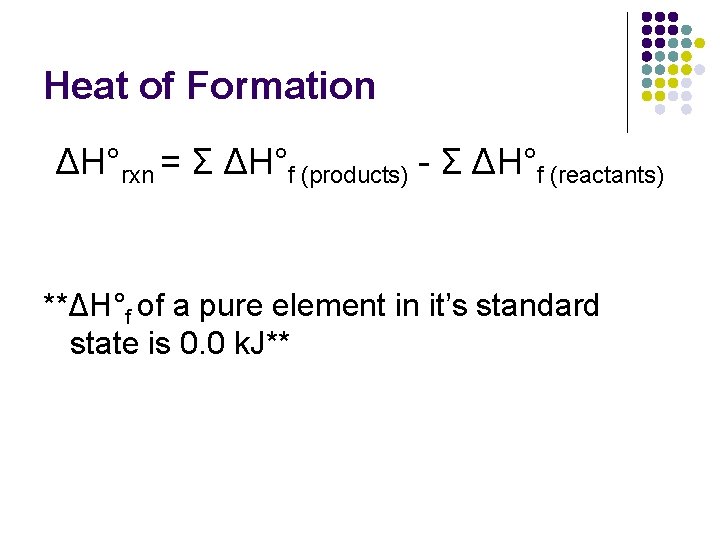
Heat of Formation l l Heat of formation-change in enthalpy that takes place when a compound is formed from it’s elements ΔH°f = standard heat of formation = the change in enthalpy that accompanies the formation of 1 mole of compound with all substances in their standard states l l Table 11. 6 in handout all in standard states: 1 atm, 298 K (25°C)

Heat of Formation ΔH°rxn = Σ ΔH°f (products) - Σ ΔH°f (reactants) **ΔH°f of a pure element in it’s standard state is 0. 0 k. J**

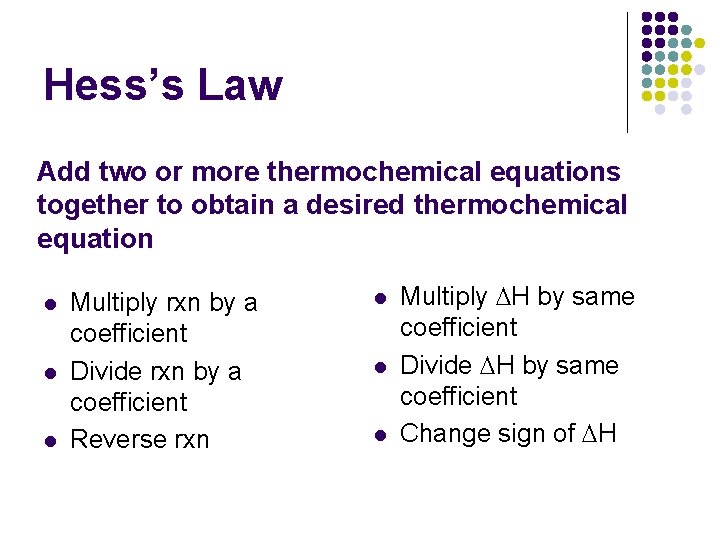
Hess’s Law Add two or more thermochemical equations together to obtain a desired thermochemical equation l l l Multiply rxn by a coefficient Divide rxn by a coefficient Reverse rxn l l l Multiply DH by same coefficient Divide DH by same coefficient Change sign of DH
 Thermochemistry is study of: *
Thermochemistry is study of: * Termokimia merupakan cabang ilmu kimia yang mempelajari
Termokimia merupakan cabang ilmu kimia yang mempelajari Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of
Thermochemistry is study of Q = m x cp x ∆t
Q = m x cp x ∆t Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of
Thermochemistry is the study of ưu thế lai là gì
ưu thế lai là gì Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế ngồi viết
Tư thế ngồi viết Chó sói
Chó sói Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu