Energetics Energy changes in chemistry Thermochemistry n What


- Slides: 17

Energetics Energy changes in chemistry.

Thermochemistry n What is thermochemistry? n Thermochemistry is the study of energy changes associated with chemical reactions.

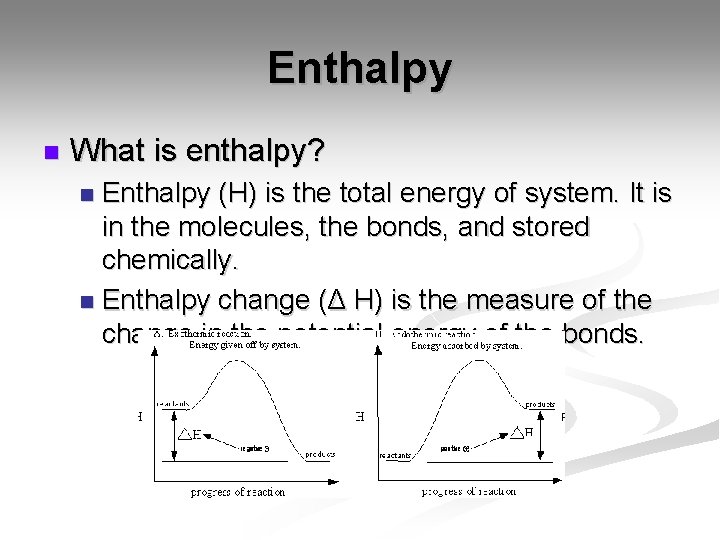
Enthalpy n What is enthalpy? Enthalpy (H) is the total energy of system. It is in the molecules, the bonds, and stored chemically. n Enthalpy change (Δ H) is the measure of the change in the potential energy of the bonds. n

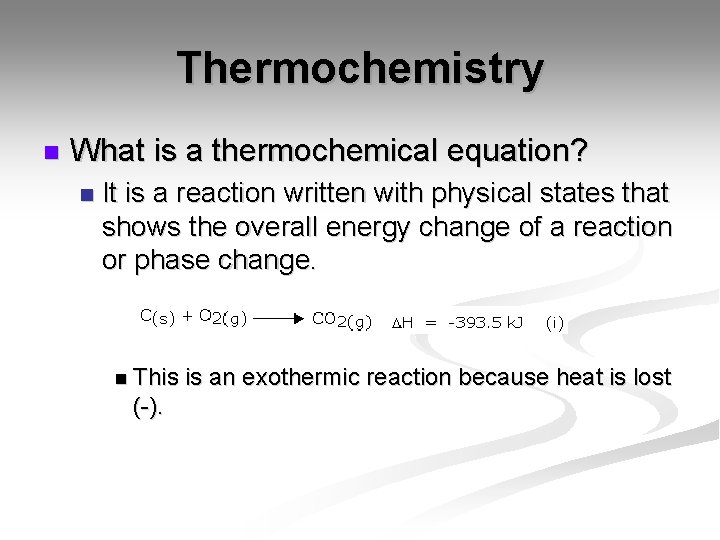
Thermochemistry n What is a thermochemical equation? n It is a reaction written with physical states that shows the overall energy change of a reaction or phase change. n This (-). is an exothermic reaction because heat is lost

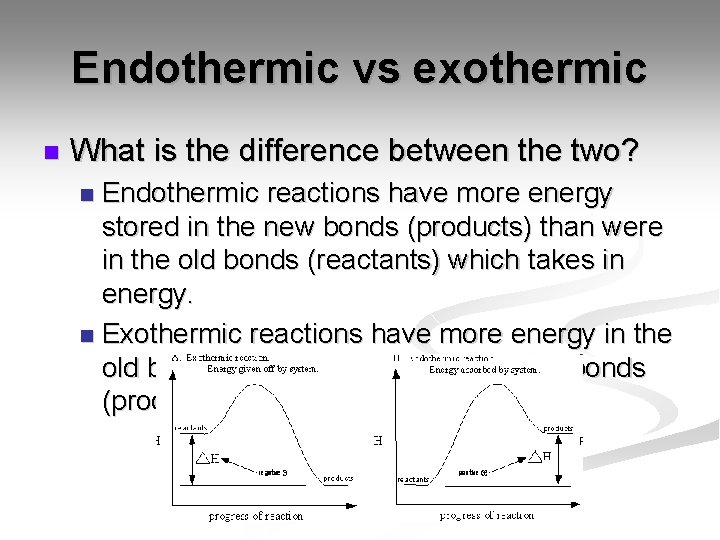
Endothermic vs exothermic n What is the difference between the two? Endothermic reactions have more energy stored in the new bonds (products) than were in the old bonds (reactants) which takes in energy. n Exothermic reactions have more energy in the old bonds (reactants) than in the new bonds (products). n

Enthalpy change n What is the value of enthalpy change for exothermic reactions? Exothermic reaction enthalpy change is negative due to Δ H = enthalpy products – enthalpy reactants. n Endothermic reaction would be the opposite because of the same equation. n

Enthalpy change n How do we show enthalpy change of a reaction? It is usually written with the equation. n + represents endothermic, - represents exothermic n It is given in the units of k. J/mol (or just k. J) because it changes with the amount of reactants and limiting reactants. n This is at thermochemical standard conditions which are 25 degrees C, 1 atm and solution concentrations of 1 mol/dm 3. n

Enthalpy change n What is the difference between heat and temperature? Temperature is the measure of the kinetic energy of the particles and does not depend on the amount present. n Heat is the measure of the total energy of a substance that increases and decreases with amount of substance. n

Enthalpy change n How do we find heat energy? Heat energy = mass x specific heat capacity x change in temperature (m x c x ΔT). n Specific heat capacity depends on the specific material involved. n

Calorimetry n What is Calorimetry? This is a technique to measure the energy change in a reaction. n Total heat = (m c ΔT)liquid + (m c ΔT)solid n

Calorimetry n When 8. 00 g of ammonium nitrate dissolves in 100. 0 cm 3 of water, the temperature dropped from 19. 0 C to 14. 5 C. What is the enthalpy of ammonium nitrate? n n n 100 g x 4. 18 J/g K x 4. 5 K 1881 J 8. 00 g/80. 06 g/mol. 100 mol 1881 J/. 100 mol 18. 8 k. J/mol

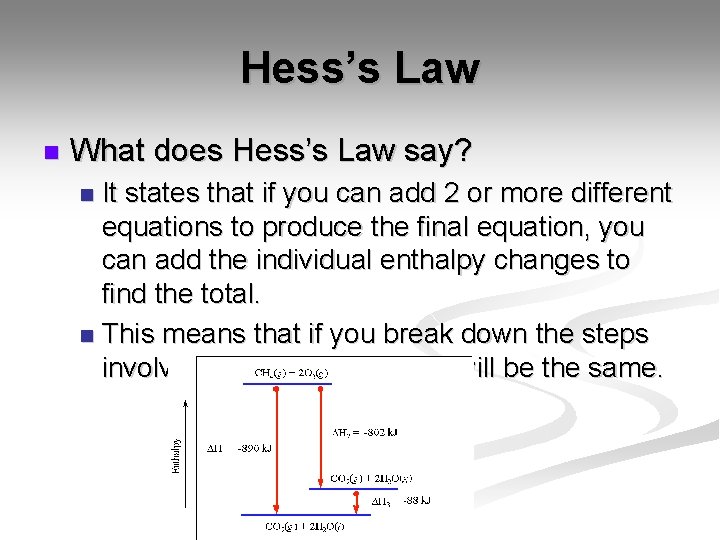
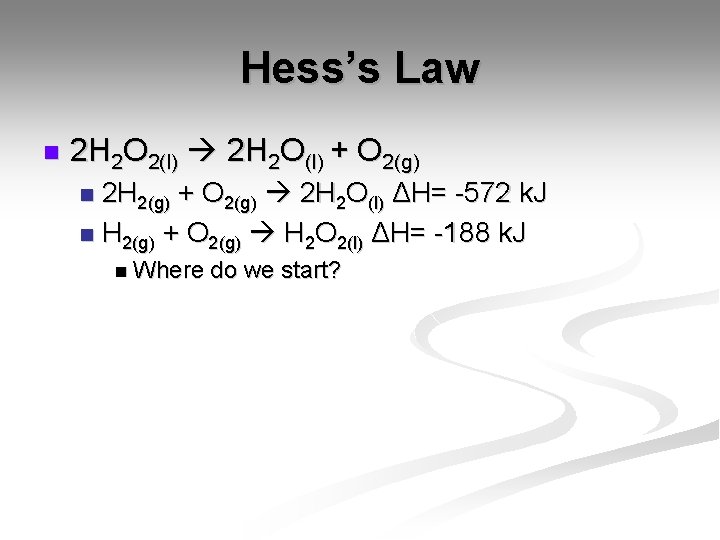
Hess’s Law n What does Hess’s Law say? It states that if you can add 2 or more different equations to produce the final equation, you can add the individual enthalpy changes to find the total. n This means that if you break down the steps involved, the overall change will be the same. n

Hess’s Law n 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) 2 H 2(g) + O 2(g) 2 H 2 O(l) ΔH= -572 k. J n H 2(g) + O 2(g) H 2 O 2(l) ΔH= -188 k. J n n Where do we start?

Reaction spontaneity n What is a spontaneous reaction? A spontaneous reaction will happen naturally. n Iron naturally rusts when left exposed. n

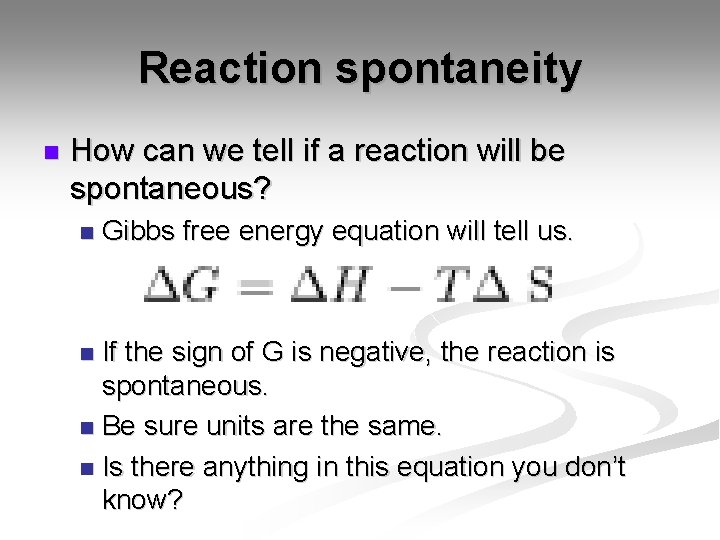
Reaction spontaneity n How can we tell if a reaction will be spontaneous? n Gibbs free energy equation will tell us. If the sign of G is negative, the reaction is spontaneous. n Be sure units are the same. n Is there anything in this equation you don’t know? n

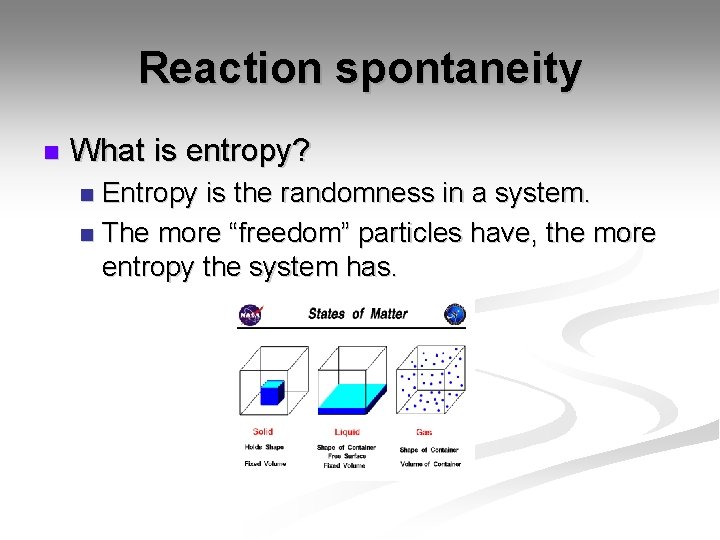
Reaction spontaneity n What is entropy? Entropy is the randomness in a system. n The more “freedom” particles have, the more entropy the system has. n

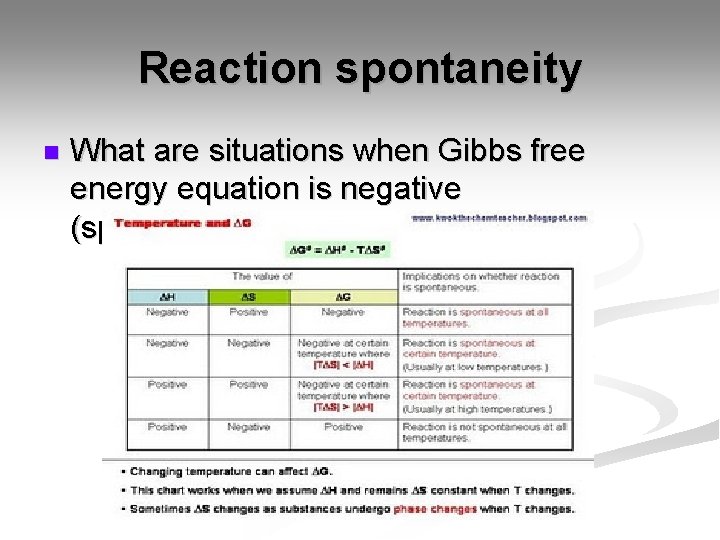
Reaction spontaneity n What are situations when Gibbs free energy equation is negative (spontaneous)?
 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermodynamics ap chemistry
Thermodynamics ap chemistry Ap chem unit 7
Ap chem unit 7 General chemistry thermochemistry
General chemistry thermochemistry Significance of hmp shunt in biochemistry
Significance of hmp shunt in biochemistry Glycolysis energetics
Glycolysis energetics Significance of glycolysis
Significance of glycolysis Sodium azide electron transport chain
Sodium azide electron transport chain Energetics power tower 180
Energetics power tower 180 Energetics of gluconeogenesis
Energetics of gluconeogenesis Building energetics
Building energetics Ib energetics
Ib energetics Energetics janesville
Energetics janesville Calorimetry 2 chemsheets answers as 1047
Calorimetry 2 chemsheets answers as 1047 Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Elizabeth mulroney
Elizabeth mulroney Example of physical change
Example of physical change