AP Chemistry Thermochemistry Chapter 6 and 17 thermodynamics








- Slides: 23

AP Chemistry Thermochemistry Chapter 6 and 17

thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes

Energy kinetic energy: energy of motion; KE = ½ mv 2 -- all particles have KE -- Thermal energy is due to the KE of particles. We measure the average KE of a collection of particles as. . . temperature. potential energy: stored energy Chemical potential energy is due to electrostatic forces between charged particles. + -- related to the specific arrangement of atoms in the substance +

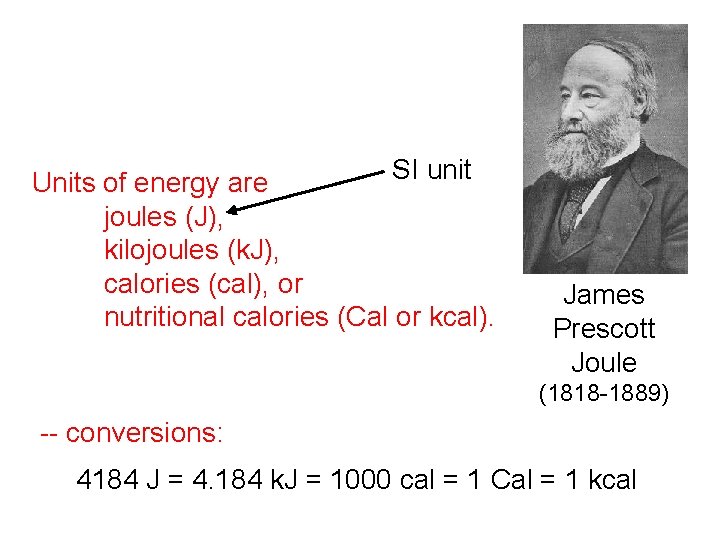
SI unit Units of energy are joules (J), kilojoules (k. J), calories (cal), or nutritional calories (Cal or kcal). James Prescott Joule (1818 -1889) -- conversions: 4184 J = 4. 184 k. J = 1000 cal = 1 Cal = 1 kcal

system: the part of the universe we are studying surroundings: everything else -- In chemistry, a closed system can exchange energy but not matter with its surroundings. -- Usually, energy is transferred to. . . …(1) change an object’s state of motion. . . or. . . (2) cause a temperature change

Work (w) is done when a force moves through a distance. W = F d Heat (q) is an amount of energy transferred from a hotter object to a colder one.

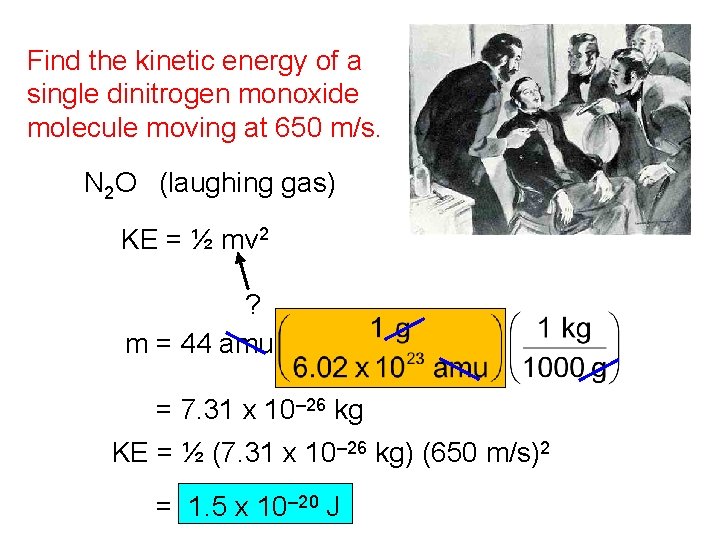
Find the kinetic energy of a single dinitrogen monoxide molecule moving at 650 m/s. N 2 O (laughing gas) KE = ½ mv 2 ? m = 44 amu = 7. 31 x 10– 26 kg KE = ½ (7. 31 x 10– 26 kg) (650 m/s)2 = 1. 5 x 10– 20 J

First Law of Thermodynamics = Law of Conservation of Energy -- Energy morphs between its various forms, but the total amount remains the same. (pretty much)

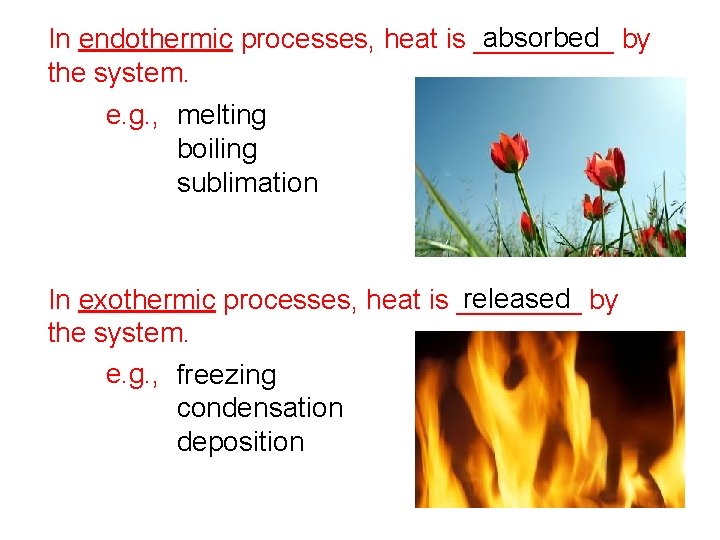
absorbed by In endothermic processes, heat is _____ the system. e. g. , melting boiling sublimation released by In exothermic processes, heat is ____ the system. e. g. , freezing condensation deposition

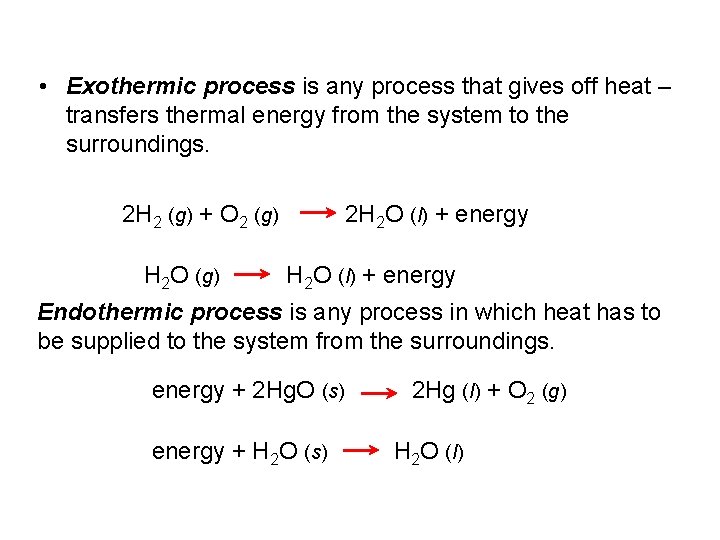
• Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) H 2 O (g) 2 H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) energy + H 2 O (s) 2 Hg (l) + O 2 (g) H 2 O (l)

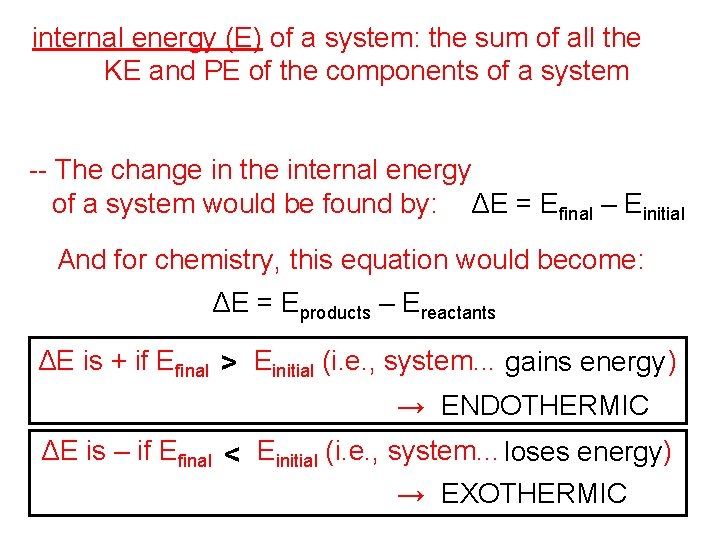
internal energy (E) of a system: the sum of all the KE and PE of the components of a system -- The change in the internal energy of a system would be found by: ΔE = Efinal – Einitial And for chemistry, this equation would become: ΔE = Eproducts – Ereactants ΔE is + if Efinal > Einitial (i. e. , system. . . gains energy ) → ENDOTHERMIC ΔE is – if Efinal < Einitial (i. e. , system. . . loses energy) → EXOTHERMIC

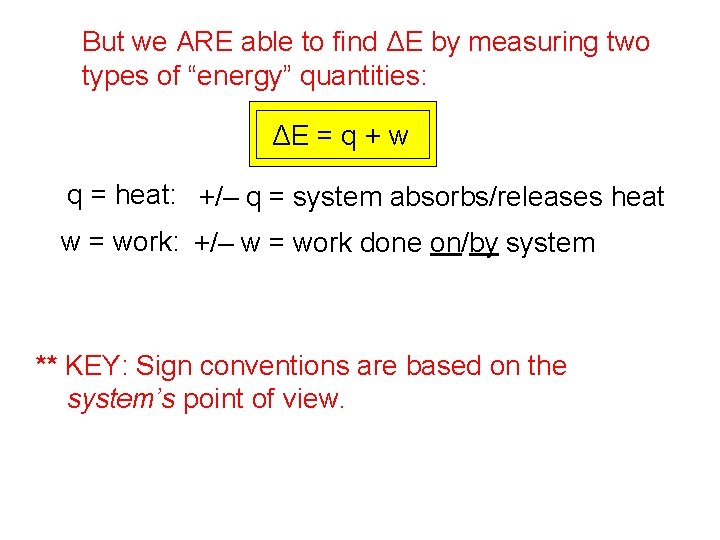
But we ARE able to find ΔE by measuring two types of “energy” quantities: ΔE = q + w q = heat: +/– q = system absorbs/releases heat w = work: +/– w = work done on/by system ** KEY: Sign conventions are based on the system’s point of view.

To go further, we must introduce the concept of enthalpy (H). -- Enthalpy (H) is defined as. . . H = E + PV where E = system’s internal energy P = pressure of the system V = volume of the system Heike Kamerlingh Onnes 1853– 1926 The Dutch physicist and Nobel laureate H. K. Onnes coined the term enthalpy, basing it on the Greek termenthalpein, which means “to warm. ”

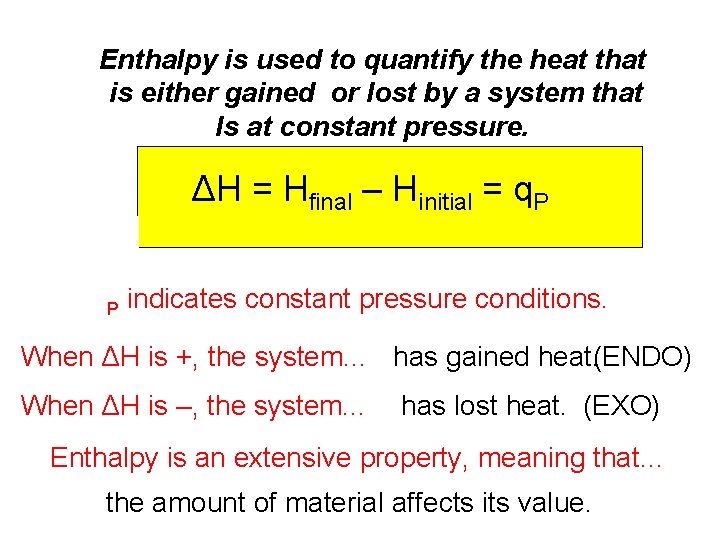
Enthalpy is used to quantify the heat that is either gained or lost by a system that Is at constant pressure. ΔH = Hfinal – Hinitial = q. P P indicates constant pressure conditions. When ΔH is +, the system. . . has gained heat. (ENDO) When ΔH is –, the system. . . has lost heat. (EXO) Enthalpy is an extensive property, meaning that… the amount of material affects its value.

In the burning of firewood at constant pressure, the enthalpy change equals the heat released. ΔH is (–) and depends on the quantity of wood burned.

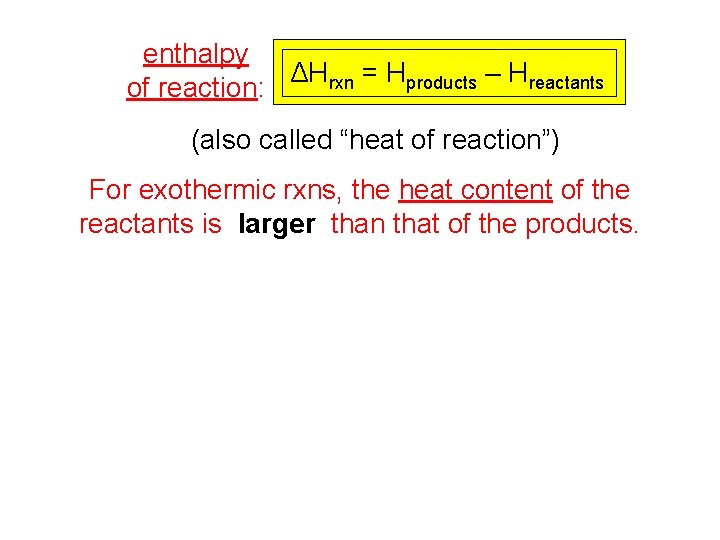
enthalpy ΔHrxn = Hproducts – Hreactants of reaction: (also called “heat of reaction”) For exothermic rxns, the heat content of the reactants is larger than that of the products.

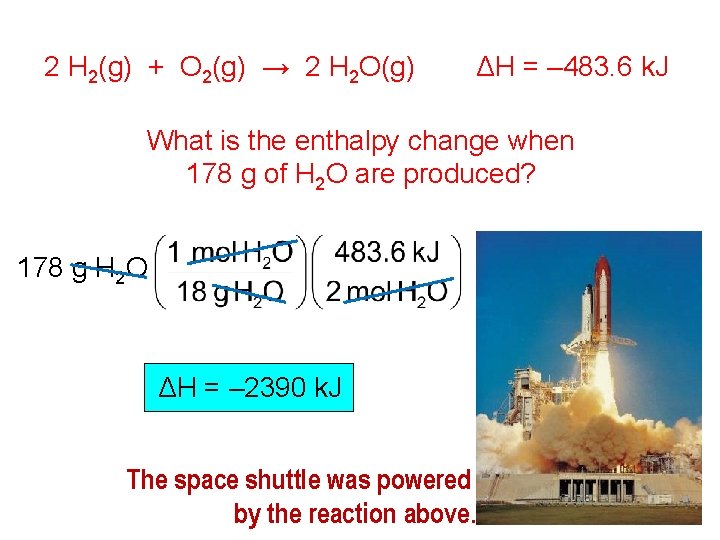
2 H 2(g) + O 2(g) → 2 H 2 O(g) ΔH = – 483. 6 k. J What is the enthalpy change when 178 g of H 2 O are produced? 178 g H 2 O ΔH = – 2390 k. J The space shuttle was powered by the reaction above.

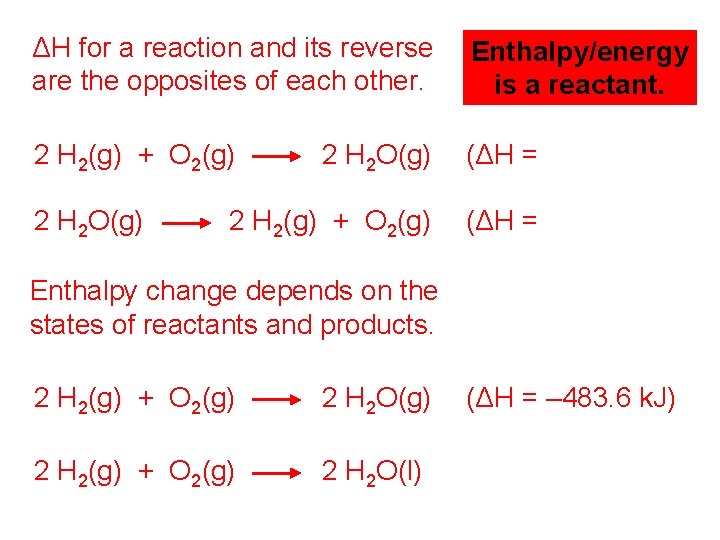
ΔH for a reaction and its reverse are the opposites of each other. Enthalpy/energy is a reactant. 2 H 2(g) + O 2(g) 2 H 2 O(g) (ΔH = – 483. 6 k. J) 2 H 2(g) + O 2(g) (ΔH = +483. 6 k. J) 2 H 2 O(g) Enthalpy change depends on the states of reactants and products. 2 H 2(g) + O 2(g) 2 H 2 O(g) (ΔH = – 483. 6 k. J) 2 H 2(g) + O 2(g) 2 H 2 O(l) (ΔH = – 571. 6 k. J)

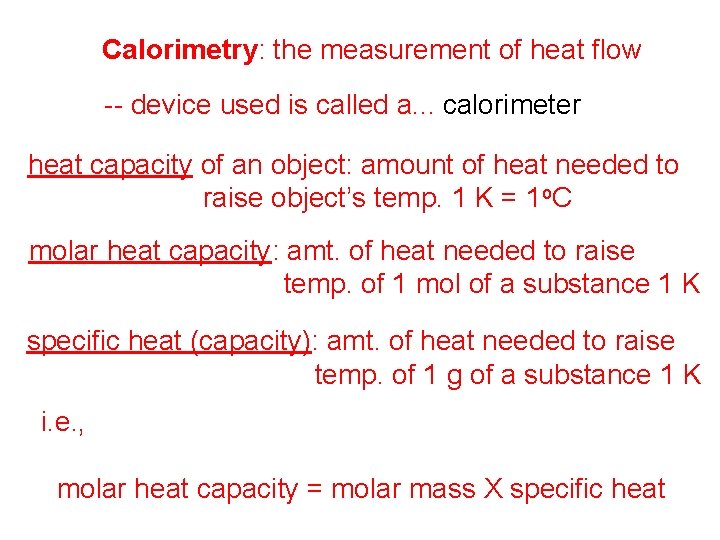
Calorimetry: the measurement of heat flow -- device used is called a. . . calorimeter heat capacity of an object: amount of heat needed to raise object’s temp. 1 K = 1 o. C molar heat capacity: amt. of heat needed to raise temp. of 1 mol of a substance 1 K specific heat (capacity): amt. of heat needed to raise temp. of 1 g of a substance 1 K i. e. , molar heat capacity = molar mass X specific heat

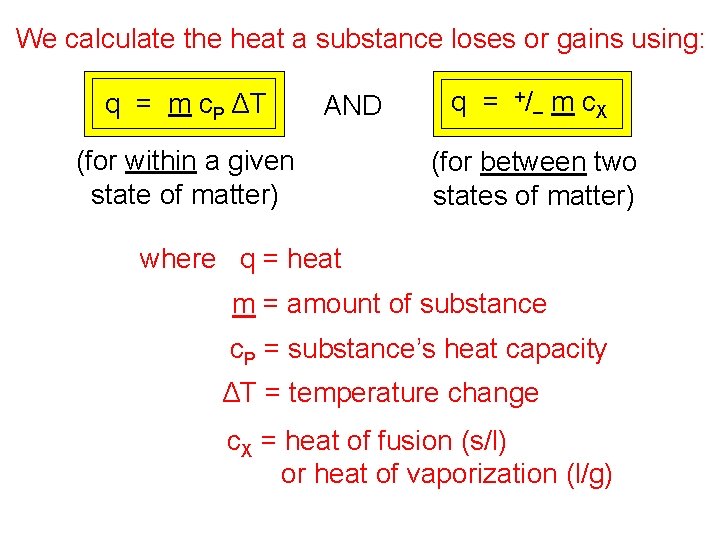
We calculate the heat a substance loses or gains using: q = m c. P ΔT AND (for within a given state of matter) q = +/– m c. X (for between two states of matter) where q = heat m = amount of substance c. P = substance’s heat capacity ΔT = temperature change c. X = heat of fusion (s/l) or heat of vaporization (l/g)

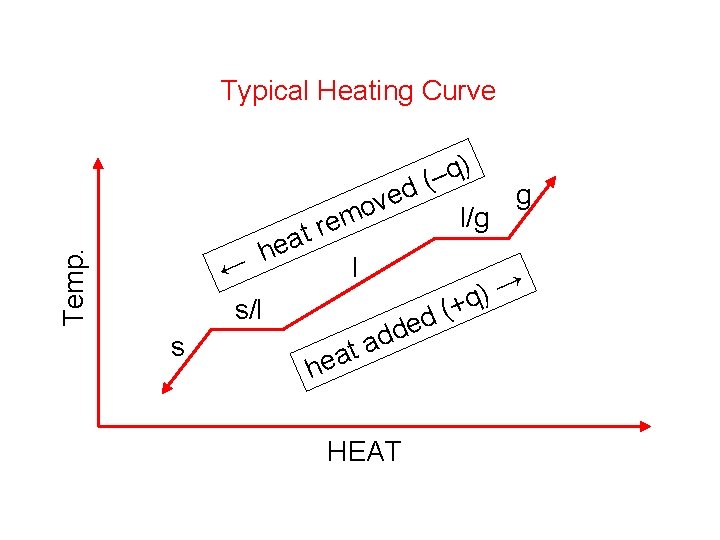
Temp. Typical Heating Curve ← s/l s t a e h ) q – ( d g ve o l/g rem l ( d e d d a t a he HEAT → ) +q

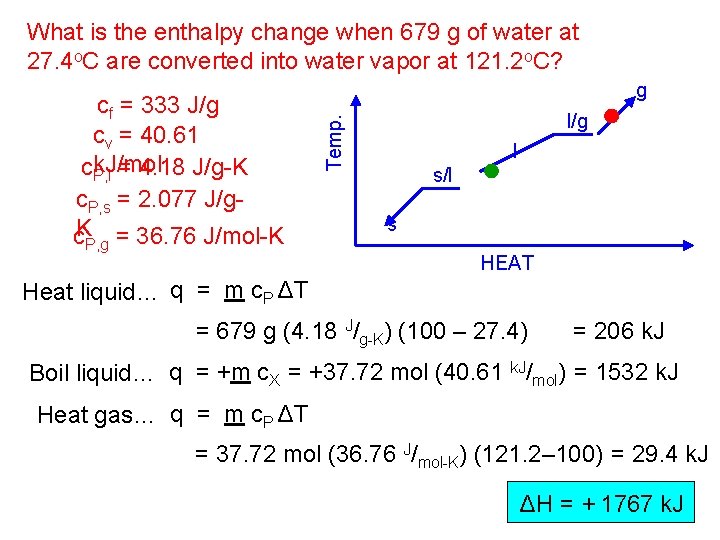
What is the enthalpy change when 679 g of water at 27. 4 o. C are converted into water vapor at 121. 2 o. C? P, g l/g Temp. cf = 333 J/g cv = 40. 61 ck. J/mol P, l = 4. 18 J/g-K c. P, s = 2. 077 J/gc. K = 36. 76 J/mol-K g l s/l s HEAT Heat liquid… q = m c. P ΔT = 679 g (4. 18 J/g-K) (100 – 27. 4) = 206 k. J Boil liquid… q = +m c. X = +37. 72 mol (40. 61 k. J/mol) = 1532 k. J Heat gas… q = m c. P ΔT = 37. 72 mol (36. 76 J/mol-K) (121. 2– 100) = 29. 4 k. J ΔH = + 1767 k. J

With a coffee-cup calorimeter, a reaction is carried out under constant pressure conditions. -- Why is the pressure constant? calorimeter isn’t sealed, atmospheric pressure is constant -- If we assume that no heat is exchanged between the system and the surroundings, then the solution must absorb any heat given off by the reaction. i. e. , qabsorbed = –qreleased the specific heat of water -- For dilute aqueous solutions, it is a safe assumption that c. P = 4. 18 J/g-K
 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chem unit 7
Ap chem unit 7 General chemistry thermochemistry
General chemistry thermochemistry Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Statistical thermodynamics is a study of
Statistical thermodynamics is a study of 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Ap chemistry thermodynamics
Ap chemistry thermodynamics 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Science chapter 5 form 3
Science chapter 5 form 3 Quiz 2: heat flow and technology
Quiz 2: heat flow and technology Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemistry
Thermochemistry Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 6
Chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Chemical engineering thermodynamics 8th solution chapter 4
Chemical engineering thermodynamics 8th solution chapter 4 Chapter 6
Chapter 6 Which of the following system is a control mass system
Which of the following system is a control mass system