CHAPTER ONE 6 Thermochemistry Chapter 1 Thermochemistry Chapter






- Slides: 49

CHAPTER ONE (6) Thermochemistry

Chapter 1 / Thermochemistry Chapter one outline Chapter One Contains: 1. 1 The Nature of Energy and Types of Energy 1. 2 Energy Changes in Chemical Reactions 1. 3 Introduction to Thermodynamics 1. 4 Enthalpy of Chemical Reactions 1. 5 Calorimetry 1. 6 Standard Enthalpy of Formation and Reaction 1. 7 Heat of Solution and Dilution 2

Chapter 1 / Thermochemistry 1. 1 The Nature of Energy and Types of Energy n Energy is the capacity to do work • Kinetic energy is the energy produced by a moving object • Radiant energy or solar energy comes from the sun and is earth’s primary energy source • Thermal energy is the energy associated with the random motion of atoms and molecules • Chemical energy is the energy stored within the bonds of chemical substances • Nuclear energy is the energy stored within the collection of neutrons and protons in the atom • Potential energy is the energy available by virtue of an object’s position 3

Chapter 1 / Thermochemistry 1. 2 Energy Changes in Chemical Reactions n Almost all chemical reactions absorb or produce (release) energy, generally in the form of heat. n Heat is the transfer of thermal energy between two bodies that are at different temperatures. (Heat always flows from a wormer object to a cooler object. ) 4

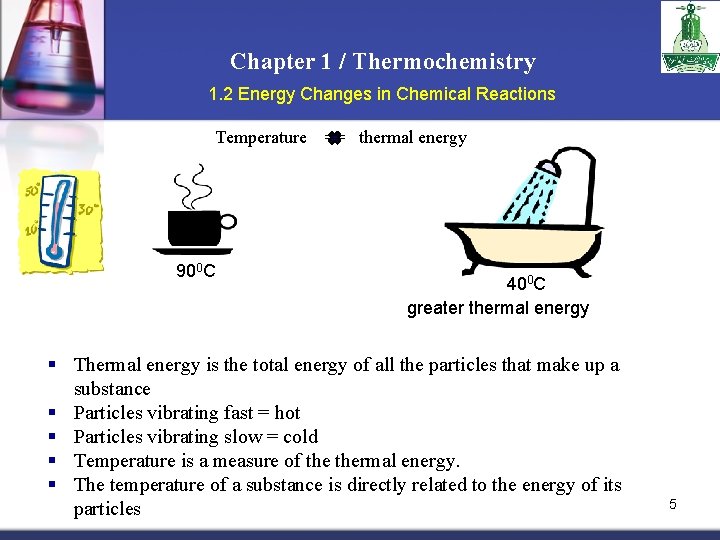
Chapter 1 / Thermochemistry 1. 2 Energy Changes in Chemical Reactions Temperature 900 C == thermal energy 400 C greater thermal energy § Thermal energy is the total energy of all the particles that make up a substance § Particles vibrating fast = hot § Particles vibrating slow = cold § Temperature is a measure of thermal energy. § The temperature of a substance is directly related to the energy of its particles 5

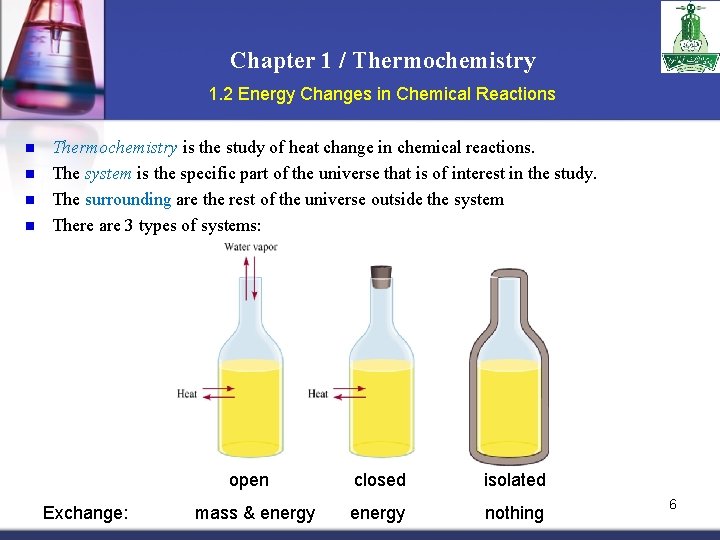
Chapter 1 / Thermochemistry 1. 2 Energy Changes in Chemical Reactions n n Thermochemistry is the study of heat change in chemical reactions. The system is the specific part of the universe that is of interest in the study. The surrounding are the rest of the universe outside the system There are 3 types of systems: open Exchange: mass & energy closed isolated energy nothing 6

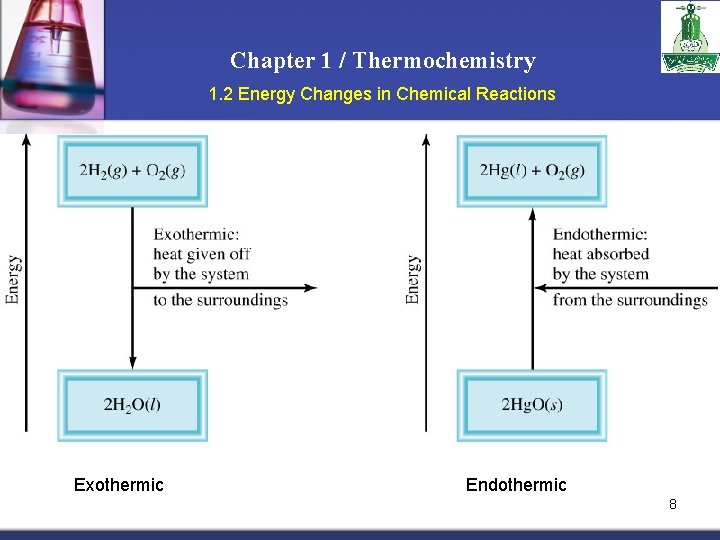
Chapter 1 / Thermochemistry 1. 2 Energy Changes in Chemical Reactions n n Essentially all chemical reactions and changes in physical state involve either release of heat or absorption of heat. Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) H 2 O (g) n 2 H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) energy + H 2 O (s) 2 Hg (l) + O 2 (g) H 2 O (l) 7

Chapter 1 / Thermochemistry 1. 2 Energy Changes in Chemical Reactions Exothermic Endothermic 8

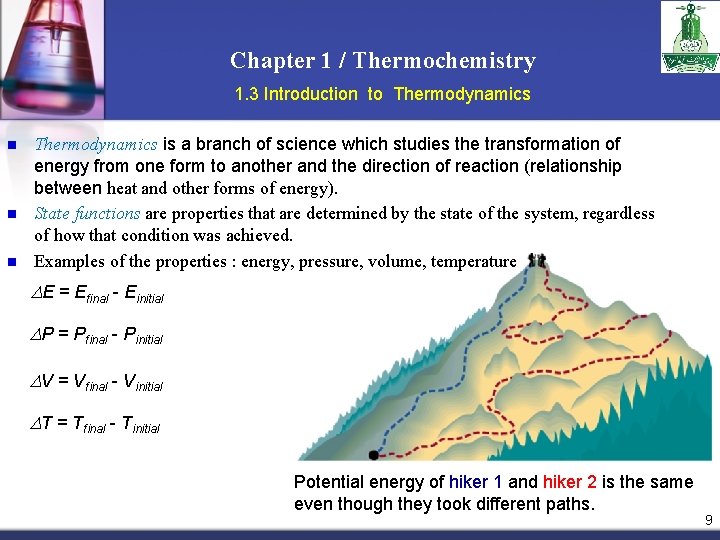
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics n n n Thermodynamics is a branch of science which studies the transformation of energy from one form to another and the direction of reaction (relationship ( between heat and other forms of energy). State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. Examples of the properties : energy, pressure, volume, temperature DE = Efinal - Einitial DP = Pfinal - Pinitial DV = Vfinal - Vinitial DT = Tfinal - Tinitial Potential energy of hiker 1 and hiker 2 is the same even though they took different paths. 9

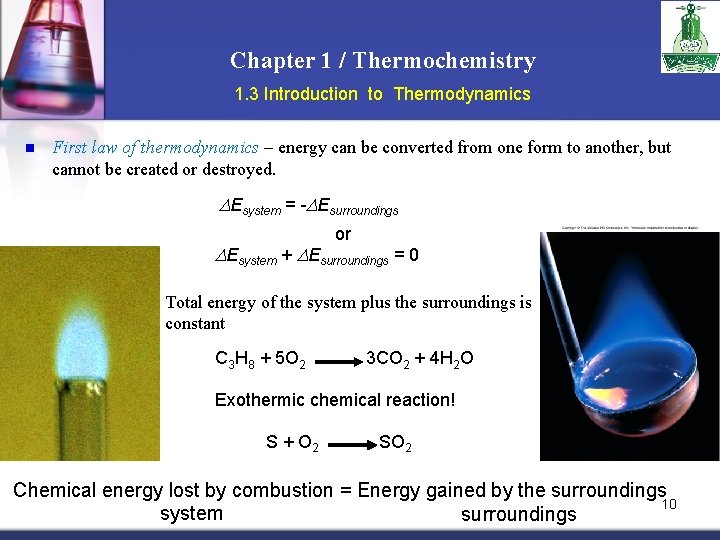
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics n First law of thermodynamics – energy can be converted from one form to another, but cannot be created or destroyed. DEsystem = -DEsurroundings or DEsystem + DEsurroundings = 0 Total energy of the system plus the surroundings is constant C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Exothermic chemical reaction! S + O 2 SO 2 Chemical energy lost by combustion = Energy gained by the surroundings 10 system surroundings

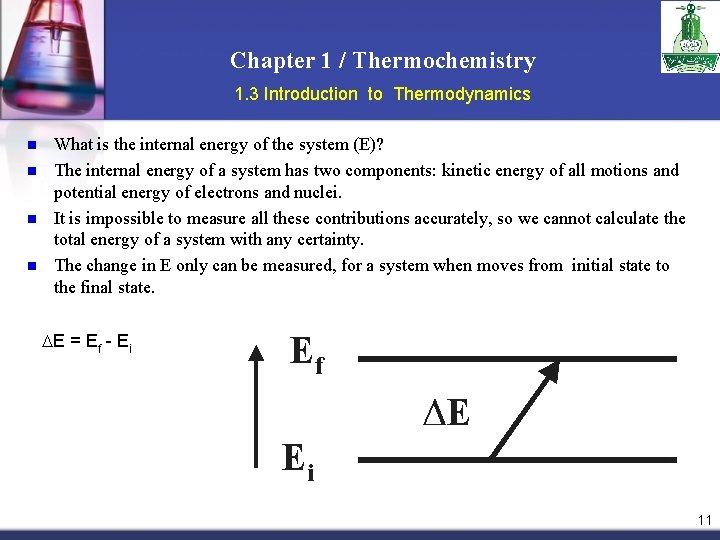
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics n n What is the internal energy of the system (E)? The internal energy of a system has two components: kinetic energy of all motions and potential energy of electrons and nuclei It is impossible to measure all these contributions accurately, so we cannot calculate the total energy of a system with any certainty. The change in E only can be measured, for a system when moves from initial state to the final state. E = Ef - Ei 11

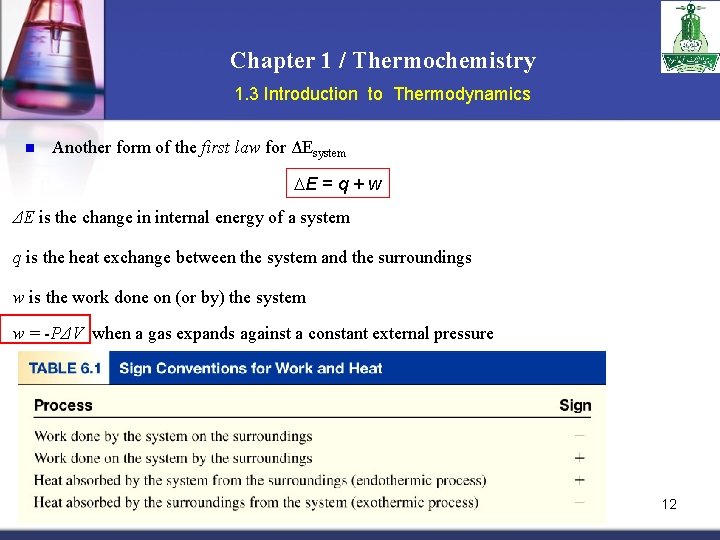
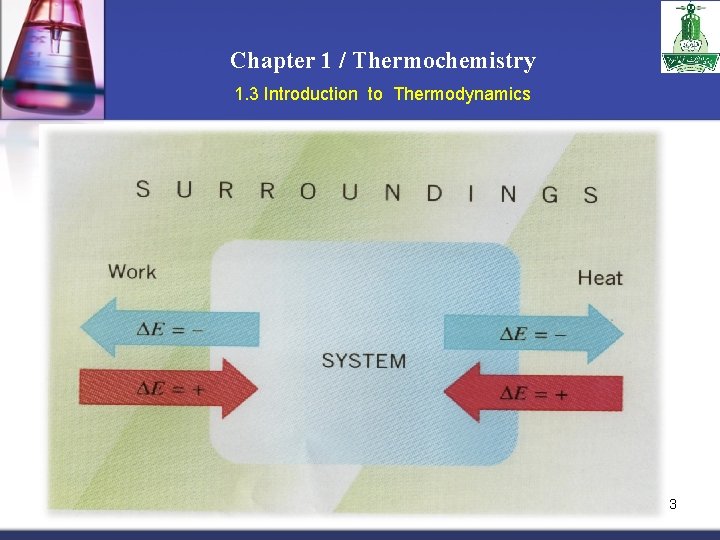
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics n Another form of the first law for ΔEsystem E = q + w ΔE is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PΔV when a gas expands against a constant external pressure 12

Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics 13

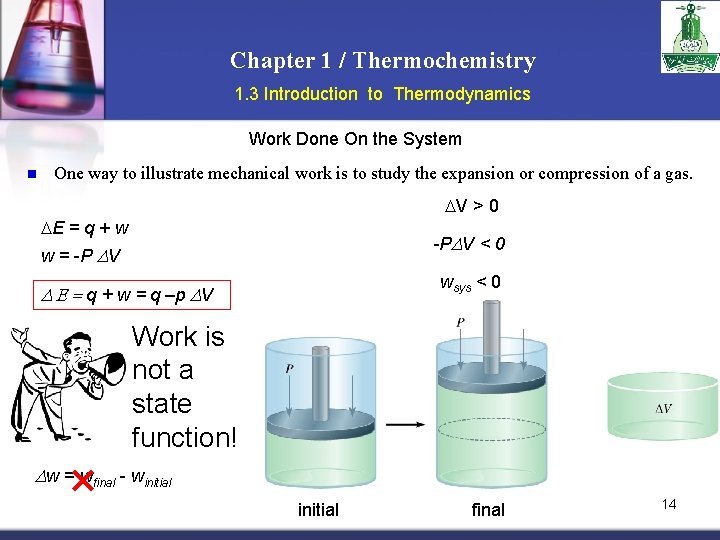
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics Work Done On the System n One way to illustrate mechanical work is to study the expansion or compression of a gas. V > 0 E = q + w -PDV < 0 w = -P DV wsys < 0 D E = q + w = q –p DV Work is not a state function! Dw = wfinal - winitial final 14

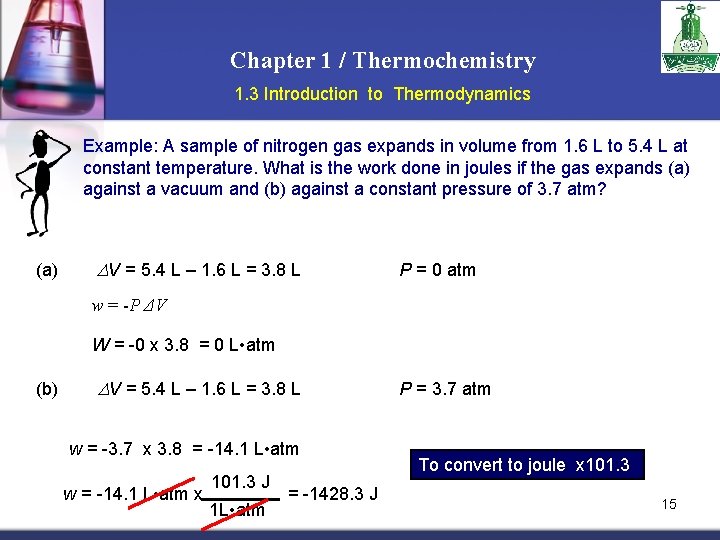
Chapter 1 / Thermochemistry 1. 3 Introduction to Thermodynamics Example: A sample of nitrogen gas expands in volume from 1. 6 L to 5. 4 L at constant temperature. What is the work done in joules if the gas expands (a) against a vacuum and (b) against a constant pressure of 3. 7 atm? (a) DV = 5. 4 L – 1. 6 L = 3. 8 L P = 0 atm w = -P ΔV W = -0 x 3. 8 = 0 L • atm (b) DV = 5. 4 L – 1. 6 L = 3. 8 L w = -3. 7 x 3. 8 = -14. 1 L • atm w = -14. 1 L • atm x 101. 3 J 1 L • atm = -1428. 3 J P = 3. 7 atm To convert to joule x 101. 3 15

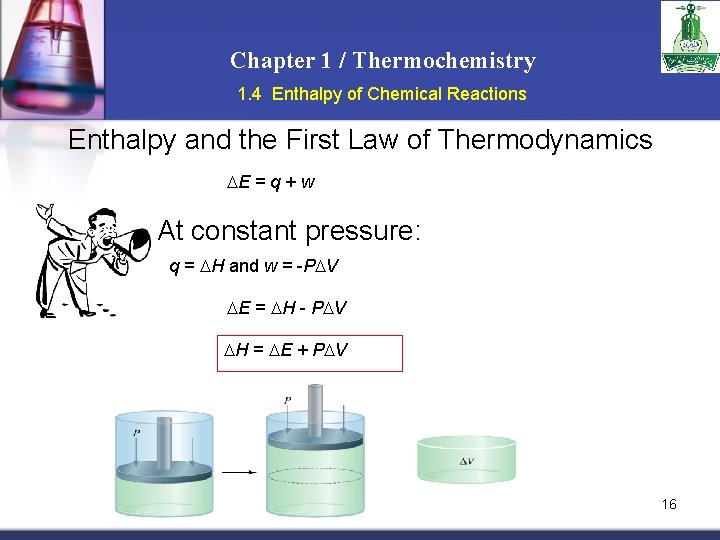
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Enthalpy and the First Law of Thermodynamics E = q + w At constant pressure: q = H and w = -P V E = H - P V H = E + P V 16

Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. H = H (products) – H (reactants) H = heat given off or absorbed during a reaction at constant pressure Hproducts < Hreactants H < 0 (Exothermic) Hproducts > Hreactants H > 0 (Endothermic) 17

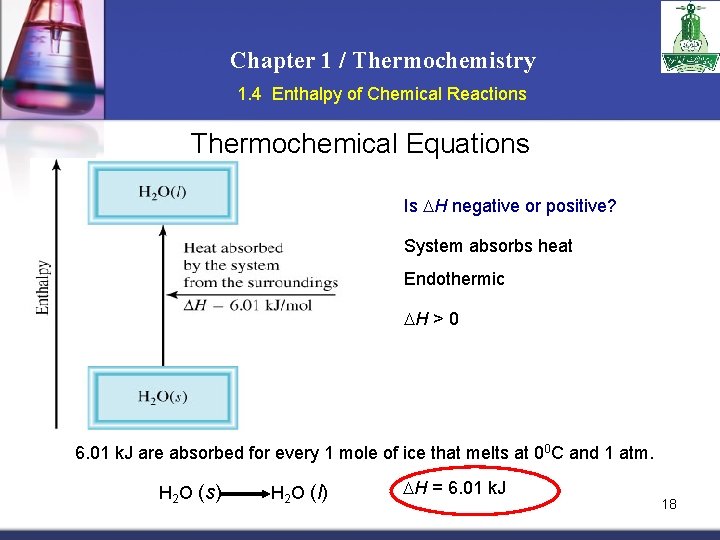
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Thermochemical Equations Is H negative or positive? System absorbs heat Endothermic H > 0 6. 01 k. J are absorbed for every 1 mole of ice that melts at 00 C and 1 atm. H 2 O (s) H 2 O (l) H = 6. 01 k. J 18

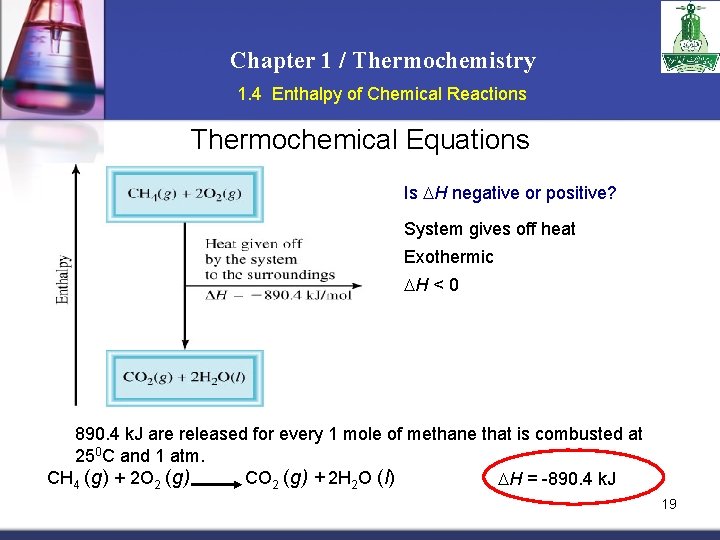
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Thermochemical Equations Is H negative or positive? System gives off heat Exothermic H < 0 890. 4 k. J are released for every 1 mole of methane that is combusted at 250 C and 1 atm. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) H = -890. 4 k. J 19

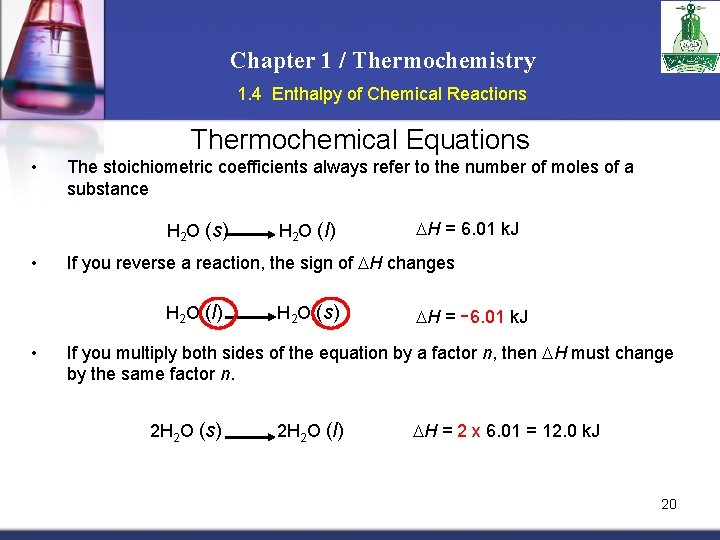
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H 2 O (s) • H = 6. 01 k. J If you reverse a reaction, the sign of H changes H 2 O (l) • H 2 O (l) H 2 O (s) H = -6. 01 k. J If you multiply both sides of the equation by a factor n, then H must change by the same factor n. 2 H 2 O (s) 2 H 2 O (l) H = 2 x 6. 01 = 12. 0 k. J 20

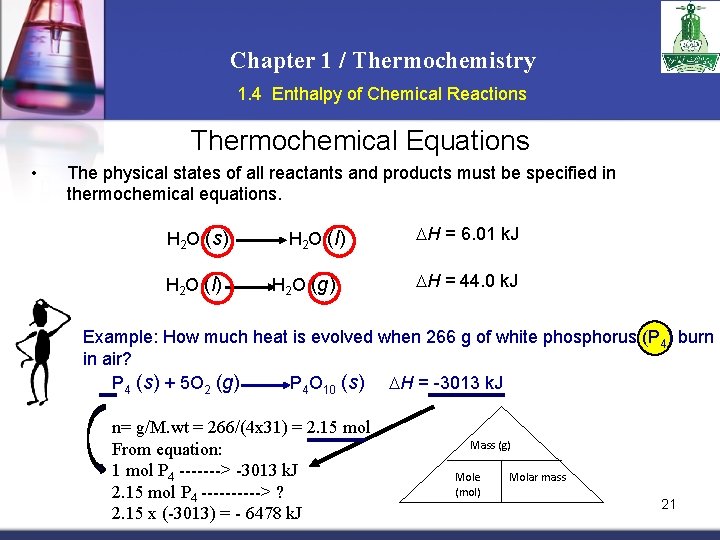
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H 2 O (s) H 2 O (l) H 2 O (g) H = 6. 01 k. J H = 44. 0 k. J Example: How much heat is evolved when 266 g of white phosphorus (P 4) burn in air? P 4 (s) + 5 O 2 (g) P 4 O 10 (s) H = -3013 k. J n= g/M. wt = 266/(4 x 31) = 2. 15 mol From equation: 1 mol P 4 -------> -3013 k. J 2. 15 mol P 4 -----> ? 2. 15 x (-3013) = - 6478 k. J Mass (g) Mole (mol) Molar mass 21

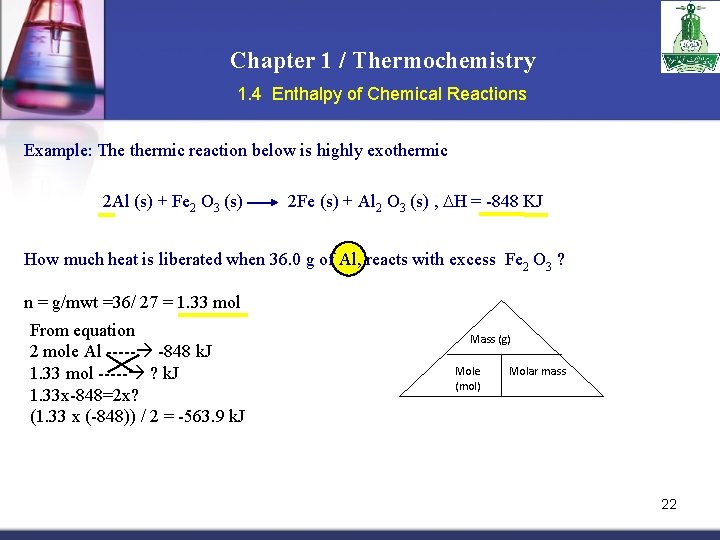
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Example: The thermic reaction below is highly exothermic 2 Al (s) + Fe 2 O 3 (s) 2 Fe (s) + Al 2 O 3 (s) , H = -848 KJ How much heat is liberated when 36. 0 g of Al, reacts with excess Fe 2 O 3 ? n = g/mwt =36/ 27 = 1. 33 mol From equation 2 mole Al ----- -848 k. J 1. 33 mol ----- ? k. J 1. 33 x-848=2 x? (1. 33 x (-848)) / 2 = -563. 9 k. J Mass (g) Mole (mol) Molar mass 22

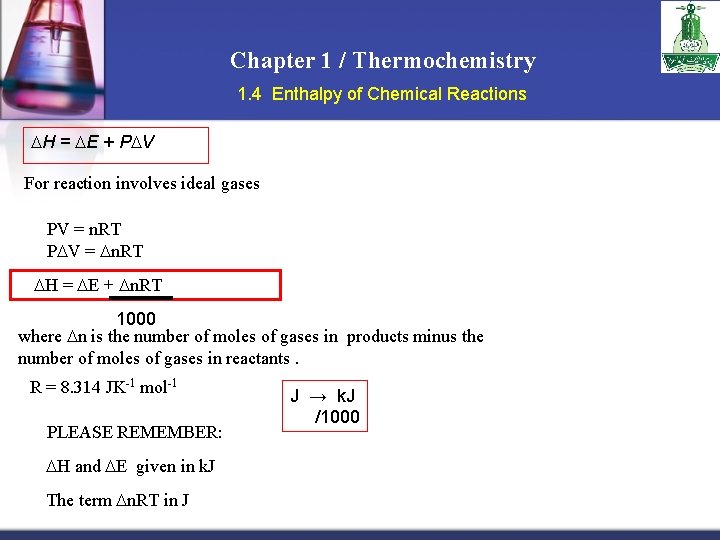
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions H = E + P V For reaction involves ideal gases v. PV = n. RT v. P V = n. RT v H = E + n. RT 1000 where n is the number of moles of gases in products minus the number of moles of gases in reactants. R = 8. 314 JK-1 mol-1 PLEASE REMEMBER: v H and E given in k. J v. The term n. RT in J J → k. J /1000

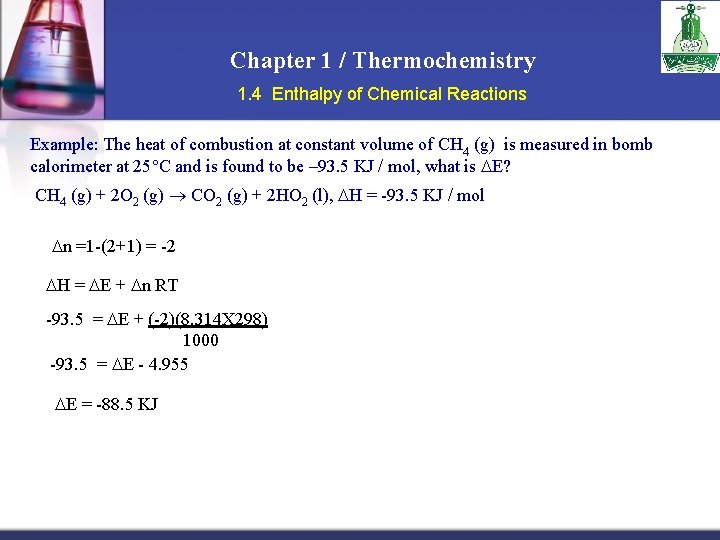
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Example: The heat of combustion at constant volume of CH 4 (g) is measured in bomb calorimeter at 25 C and is found to be – 93. 5 KJ / mol, what is E? CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 HO 2 (l), H = -93. 5 KJ / mol v n =1 -(2+1) = -2 v H = E + n RT v-93. 5 = E + (-2)(8. 314 X 298) 1000 v-93. 5 = E - 4. 955 E = -88. 5 KJ

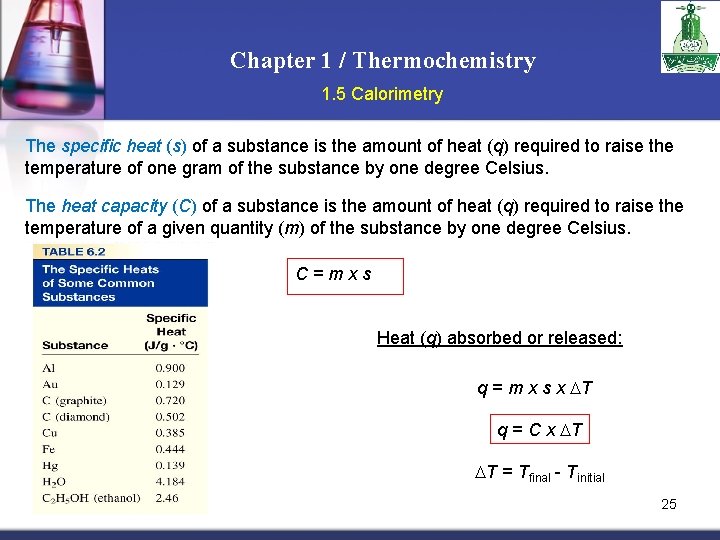
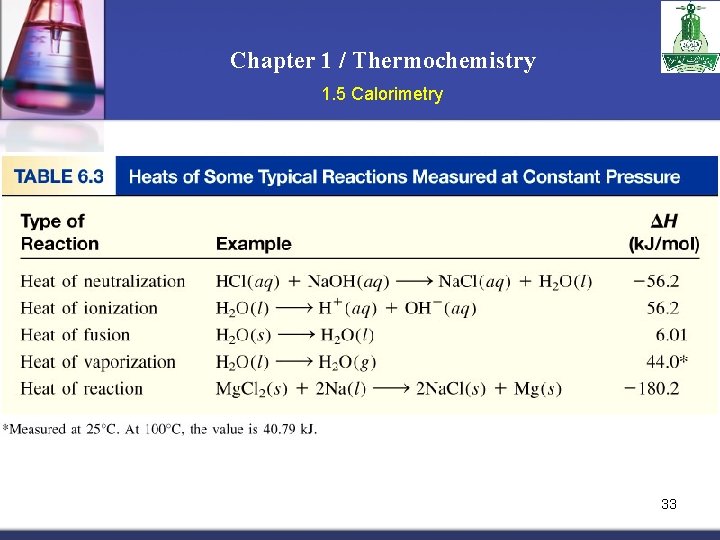
Chapter 1 / Thermochemistry 1. 5 Calorimetry The specific heat (s) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C=mxs Heat (q) absorbed or released: q = m x s x T q = C x T T = Tfinal - Tinitial 25

Chapter 1 / Thermochemistry 1. 5 Calorimetry Example: How much heat is given off when an 869 g iron bar cools from 940 C to 50 C if the specific heat of iron is 0. 444 J/g. °C? T = Tfinal – Tinitial = 50 C – 940 C = -890 C q = m x s x T = 869 x 0. 444 x – 89 = -34339 J= -34. 339 k. J 26

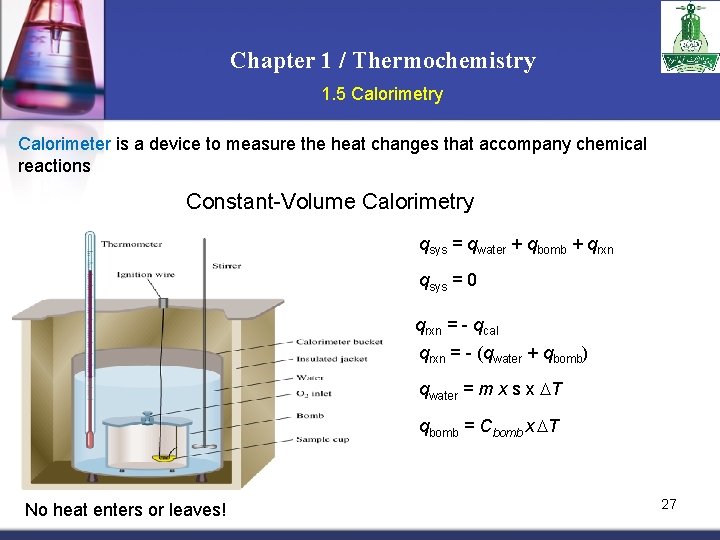
Chapter 1 / Thermochemistry 1. 5 Calorimetry Calorimeter is a device to measure the heat changes that accompany chemical reactions Constant-Volume Calorimetry qsys = qwater + qbomb + qrxn qsys = 0 qrxn = - qcal qrxn = - (qwater + qbomb) qwater = m x s x T qbomb = Cbomb x T No heat enters or leaves! 27

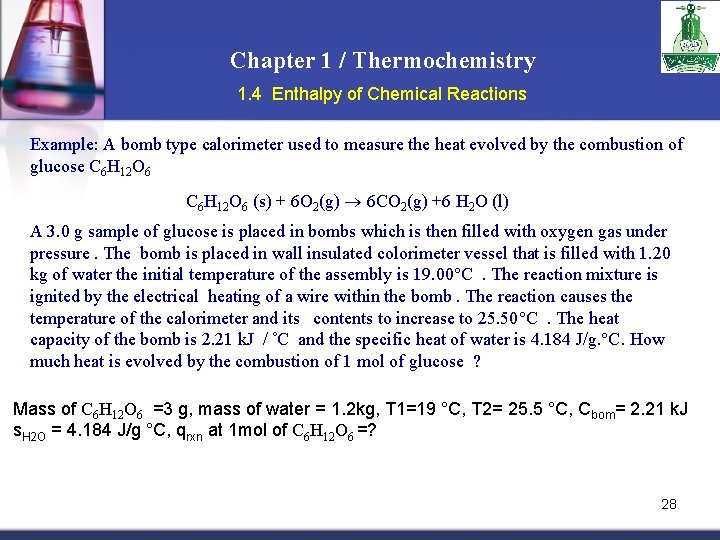
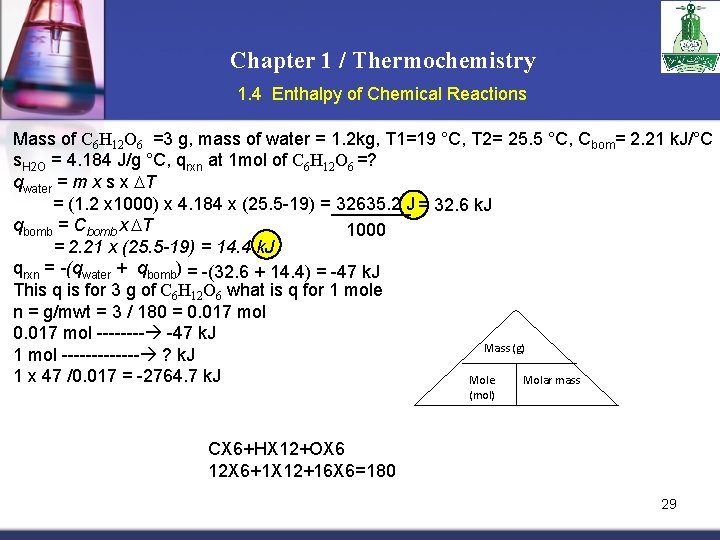
Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Example: A bomb type calorimeter used to measure the heat evolved by the combustion of glucose C 6 H 12 O 6 (s) + 6 O 2(g) 6 CO 2(g) +6 H 2 O (l) A 3. 0 g sample of glucose is placed in bombs which is then filled with oxygen gas under pressure. The bomb is placed in wall insulated colorimeter vessel that is filled with 1. 20 kg of water the initial temperature of the assembly is 19. 00 C. The reaction mixture is ignited by the electrical heating of a wire within the bomb. The reaction causes the temperature of the calorimeter and its contents to increase to 25. 50 C. The heat capacity of the bomb is 2. 21 k. J / C and the specific heat of water is 4. 184 J/g. °C. How much heat is evolved by the combustion of 1 mol of glucose ? Mass of C 6 H 12 O 6 =3 g, mass of water = 1. 2 kg, T 1=19 °C, T 2= 25. 5 °C, Cbom= 2. 21 k. J s. H 2 O = 4. 184 J/g °C, qrxn at 1 mol of C 6 H 12 O 6 =? 28

Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Mass of C 6 H 12 O 6 =3 g, mass of water = 1. 2 kg, T 1=19 °C, T 2= 25. 5 °C, Cbom= 2. 21 k. J/°C s. H 2 O = 4. 184 J/g °C, qrxn at 1 mol of C 6 H 12 O 6 =? qwater = m x s x T = (1. 2 x 1000) x 4. 184 x (25. 5 -19) = 32635. 2 J = 32. 6 k. J qbomb = Cbomb x T 1000 = 2. 21 x (25. 5 -19) = 14. 4 k. J qrxn = -(qwater + qbomb) = -(32. 6 + 14. 4) = -47 k. J This q is for 3 g of C 6 H 12 O 6 what is q for 1 mole n = g/mwt = 3 / 180 = 0. 017 mol ---- -47 k. J Mass (g) 1 mol ------- ? k. J 1 x 47 /0. 017 = -2764. 7 k. J Mole Molar mass (mol) CX 6+HX 12+OX 6 12 X 6+1 X 12+16 X 6=180 29

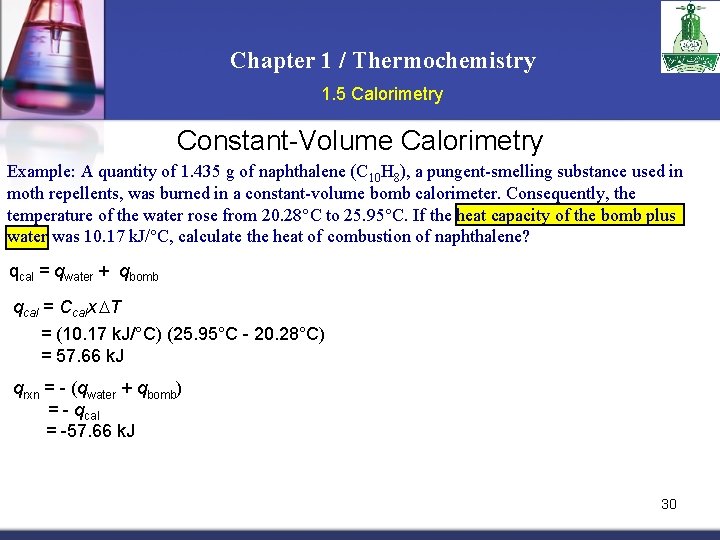
Chapter 1 / Thermochemistry 1. 5 Calorimetry Constant-Volume Calorimetry Example: A quantity of 1. 435 g of naphthalene (C 10 H 8), a pungent-smelling substance used in moth repellents, was burned in a constant-volume bomb calorimeter. Consequently, the temperature of the water rose from 20. 28°C to 25. 95°C. If the heat capacity of the bomb plus water was 10. 17 k. J/°C, calculate the heat of combustion of naphthalene? qcal = qwater + qbomb qcal = Ccalx T = (10. 17 k. J/°C) (25. 95°C - 20. 28°C) = 57. 66 k. J qrxn = - (qwater + qbomb) = - qcal = -57. 66 k. J 30

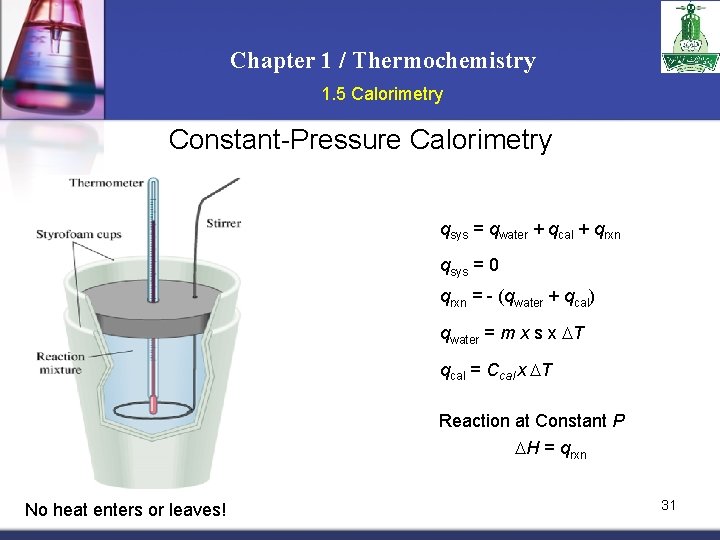
Chapter 1 / Thermochemistry 1. 5 Calorimetry Constant-Pressure Calorimetry qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = m x s x T qcal = Ccal x T Reaction at Constant P H = qrxn No heat enters or leaves! 31

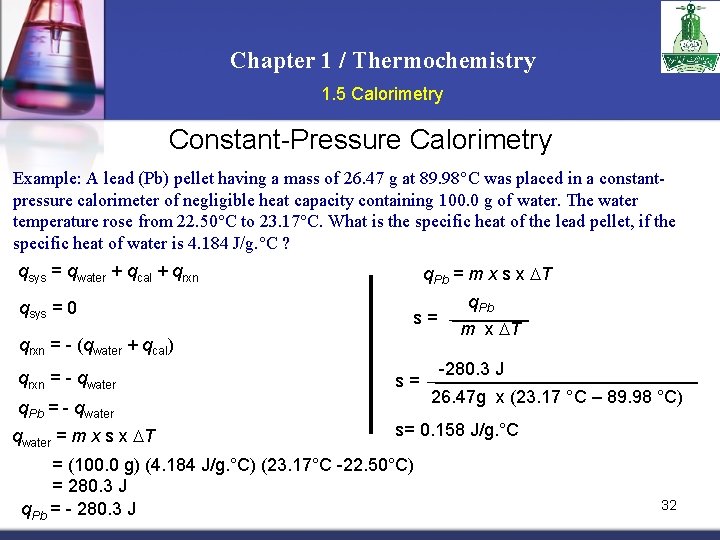
Chapter 1 / Thermochemistry 1. 5 Calorimetry Constant-Pressure Calorimetry Example: A lead (Pb) pellet having a mass of 26. 47 g at 89. 98°C was placed in a constantpressure calorimeter of negligible heat capacity containing 100. 0 g of water. The water temperature rose from 22. 50°C to 23. 17°C. What is the specific heat of the lead pellet, if the specific heat of water is 4. 184 J/g. °C ? qsys = qwater + qcal + qrxn qsys = 0 q. Pb = m x s x T s= qrxn = - (qwater + qcal) qrxn = - qwater q. Pb = - qwater = m x s x T s= q. Pb m x T -280. 3 J 26. 47 g x (23. 17 °C – 89. 98 °C) s= 0. 158 J/g. °C = (100. 0 g) (4. 184 J/g. °C) (23. 17°C -22. 50°C) = 280. 3 J q. Pb = - 280. 3 J 32

Chapter 1 / Thermochemistry 1. 5 Calorimetry 33

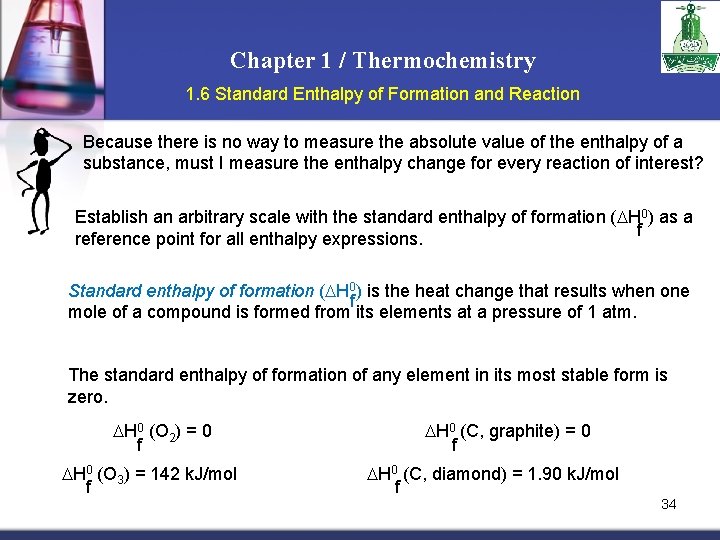
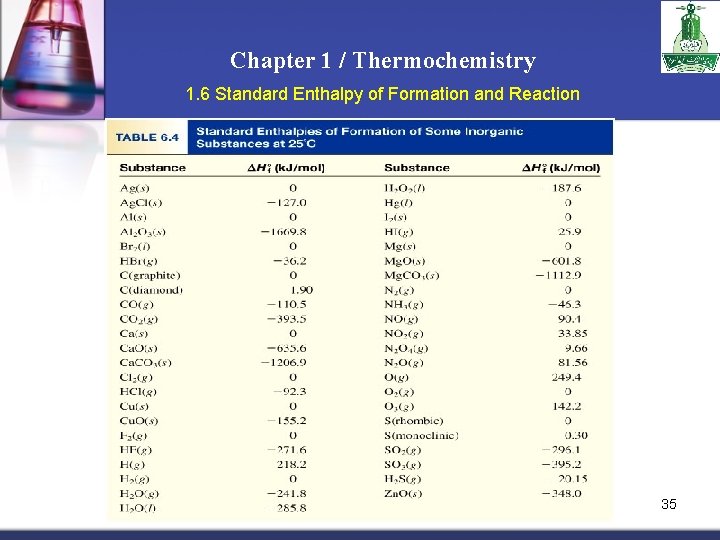
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Because there is no way to measure the absolute value of the enthalpy of a substance, must I measure the enthalpy change for every reaction of interest? Establish an arbitrary scale with the standard enthalpy of formation ( H 0) as a f reference point for all enthalpy expressions. Standard enthalpy of formation ( H 0) is the heat change that results when one f mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. H 0 (O 2) = 0 f H 0 (O 3) = 142 k. J/mol f H 0 (C, graphite) = 0 f H 0 (C, diamond) = 1. 90 k. J/mol f 34

Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction 35

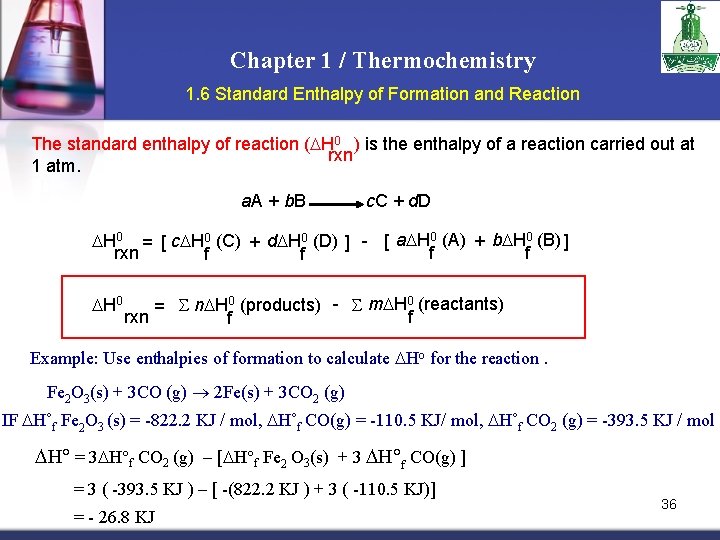
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction The standard enthalpy of reaction ( H 0 ) is the enthalpy of a reaction carried out at rxn 1 atm. a. A + b. B c. C + d. D H 0 = [ c H 0 (C) + d H 0 (D) ] - [ a H 0 (A) + b H 0 (B) ] rxn f f H 0 = n H 0 (products) - m H 0 (reactants) rxn f f Example: Use enthalpies of formation to calculate Ho for the reaction. Fe 2 O 3(s) + 3 CO (g) 2 Fe(s) + 3 CO 2 (g) IF H˚f Fe 2 O 3 (s) = -822. 2 KJ / mol, H˚f CO(g) = -110. 5 KJ/ mol, H˚f CO 2 (g) = -393. 5 KJ / mol H = 3 H f CO 2 (g) – [ H f Fe 2 O 3(s) + 3 H f CO(g) ] = 3 ( -393. 5 KJ ) – [ -(822. 2 KJ ) + 3 ( -110. 5 KJ)] = - 26. 8 KJ 36

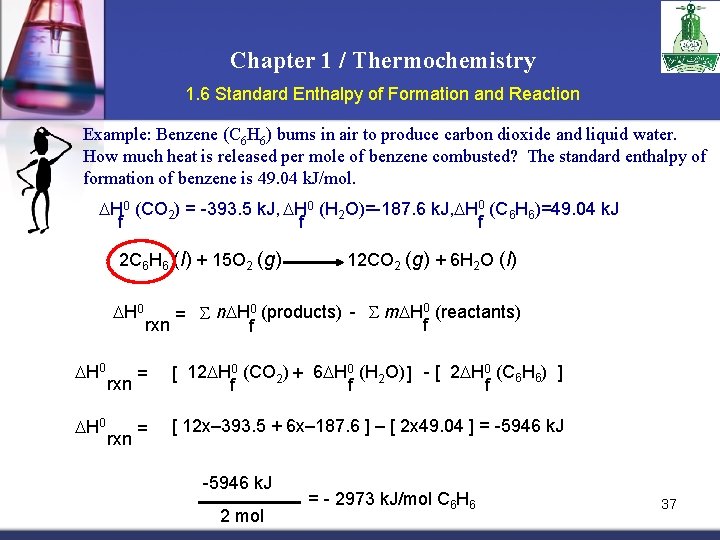
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Example: Benzene (C 6 H 6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49. 04 k. J/mol. H 0 (CO 2) = -393. 5 k. J, H 0 (H 2 O)=-187. 6 k. J, H 0 (C 6 H 6)=49. 04 k. J f f f 2 C 6 H 6 (l) + 15 O 2 (g) H 0 H 0 rxn 12 CO 2 (g) + 6 H 2 O (l) = n H 0 (products) - m H 0 (reactants) rxn f f = [ 12 H 0 (CO 2) + 6 H 0 (H 2 O) ] - [ 2 H 0 (C 6 H 6) ] f f f = [ 12 x– 393. 5 + 6 x– 187. 6 ] – [ 2 x 49. 04 ] = -5946 k. J 2 mol = - 2973 k. J/mol C 6 H 6 37

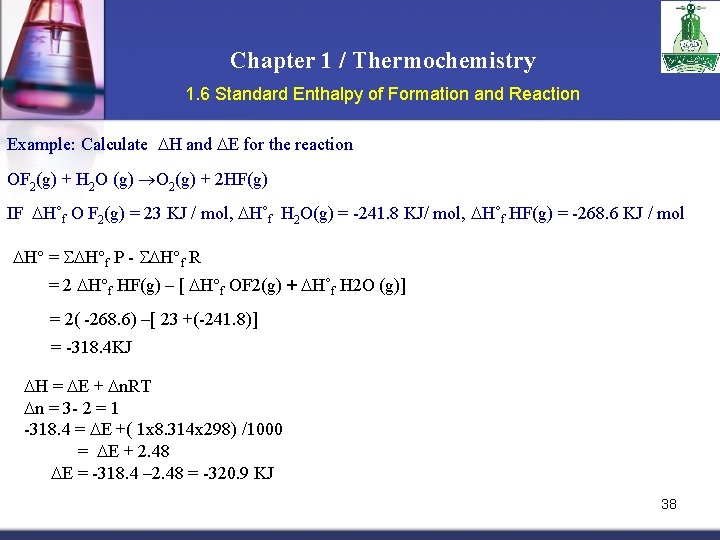
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Example: Calculate H and E for the reaction OF 2(g) + H 2 O (g) O 2(g) + 2 HF(g) IF H˚f O F 2(g) = 23 KJ / mol, H˚f H 2 O(g) = -241. 8 KJ/ mol, H˚f HF(g) = -268. 6 KJ / mol H° = H°f P - H°f R = 2 H°f HF(g) – [ H°f OF 2(g) + H˚f H 2 O (g)] = 2( -268. 6) –[ 23 +(-241. 8)] = -318. 4 KJ H = E + n. RT n = 3 - 2 = 1 -318. 4 = E +( 1 x 8. 314 x 298) /1000 = E + 2. 48 E = -318. 4 – 2. 48 = -320. 9 KJ 38

Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. ) 39

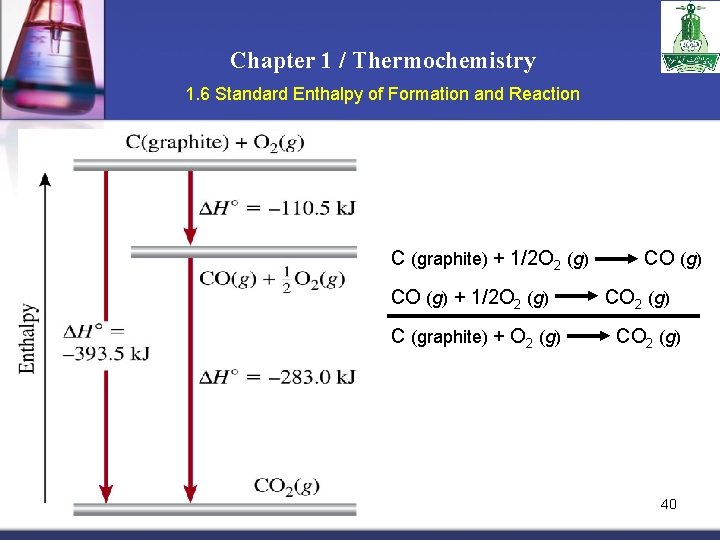
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction C (graphite) + 1/2 O 2 (g) CO (g) + 1/2 O 2 (g) C (graphite) + O 2 (g) CO 2 (g) 40

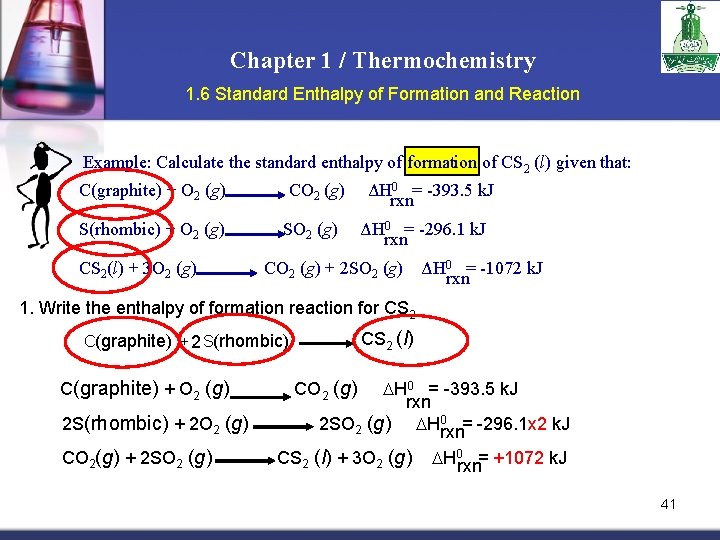
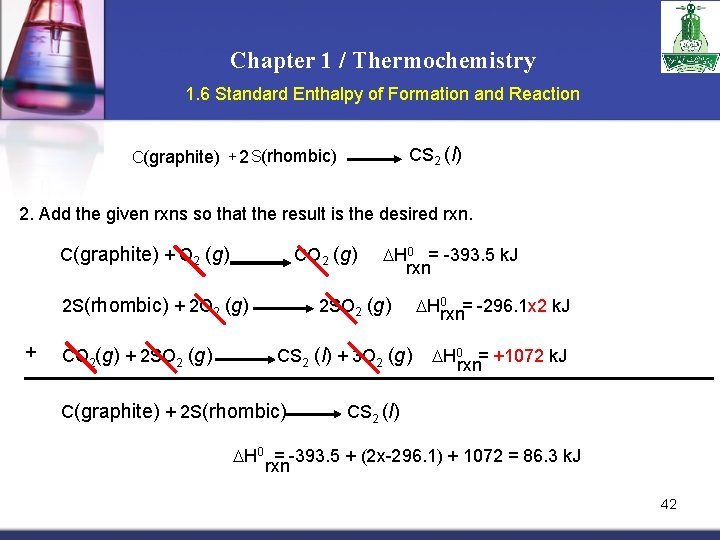
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Example: Calculate the standard enthalpy of formation of CS 2 (l) given that: C(graphite) + O 2 (g) S(rhombic) + O 2 (g) CS 2(l) + 3 O 2 (g) CO 2 (g) SO 2 (g) ΔH 0 = -393. 5 k. J rxn ΔH 0 = -296. 1 k. J rxn CO 2 (g) + 2 SO 2 (g) ΔH 0 = -1072 k. J rxn 1. Write the enthalpy of formation reaction for CS 2 (l) C(graphite) + 2 S(rhombic) C(graphite) + O 2 (g) 2 S(rhombic) + 2 O 2 (g) CO 2(g) + 2 SO 2 (g) CO 2 (g) H 0 = -393. 5 k. J rxn 2 SO 2 (g) H 0 rxn= -296. 1 x 2 k. J CS 2 (l) + 3 O 2 (g) H 0 rxn= +1072 k. J 41

Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction CS 2 (l) C(graphite) + 2 S(rhombic) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O 2 (g) CO 2 (g) 2 S(rhombic) + 2 O 2 (g) + CO 2(g) + 2 SO 2 (g) H 0 = -393. 5 k. J rxn 2 SO 2 (g) CS 2 (l) + 3 O 2 (g) C(graphite) + 2 S(rhombic) H 0 rxn= -296. 1 x 2 k. J H 0 rxn= +1072 k. J CS 2 (l) H 0 = -393. 5 + (2 x-296. 1) + 1072 = 86. 3 k. J rxn 42

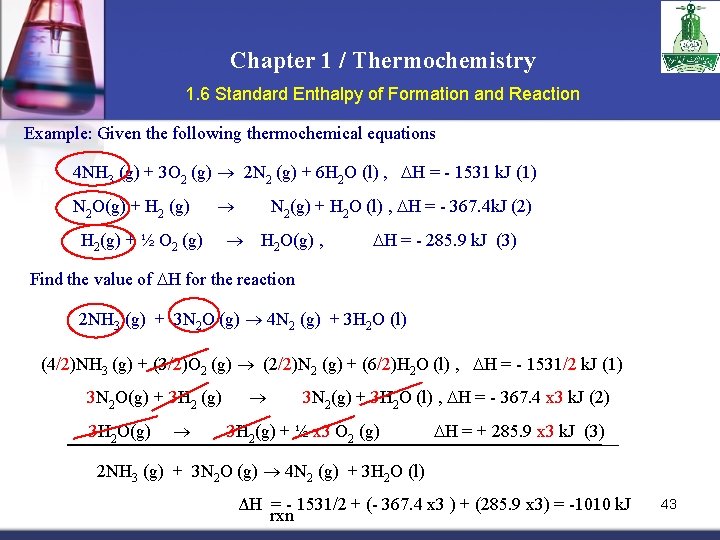
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Example: Given the following thermochemical equations 4 NH 3 (g) + 3 O 2 (g) 2 N 2 (g) + 6 H 2 O (l) , H = - 1531 k. J (1) N 2 O(g) + H 2 (g) H 2(g) + ½ O 2 (g) N 2(g) + H 2 O (l) , H = - 367. 4 k. J (2) H 2 O(g) , H = - 285. 9 k. J (3) Find the value of H for the reaction 2 NH 3 (g) + 3 N 2 O (g) 4 N 2 (g) + 3 H 2 O (l) (4/2)NH 3 (g) + (3/2)O 2 (g) (2/2)N 2 (g) + (6/2)H 2 O (l) , H = - 1531/2 k. J (1) 3 N 2 O(g) + 3 H 2 (g) 3 H 2 O(g) 3 N 2(g) + 3 H 2 O (l) , H = - 367. 4 x 3 k. J (2) 3 H 2(g) + ½ x 3 O 2 (g) H = + 285. 9 x 3 k. J (3) 2 NH 3 (g) + 3 N 2 O (g) 4 N 2 (g) + 3 H 2 O (l) ΔH = - 1531/2 + (- 367. 4 x 3 ) + (285. 9 x 3) = -1010 k. J rxn 43

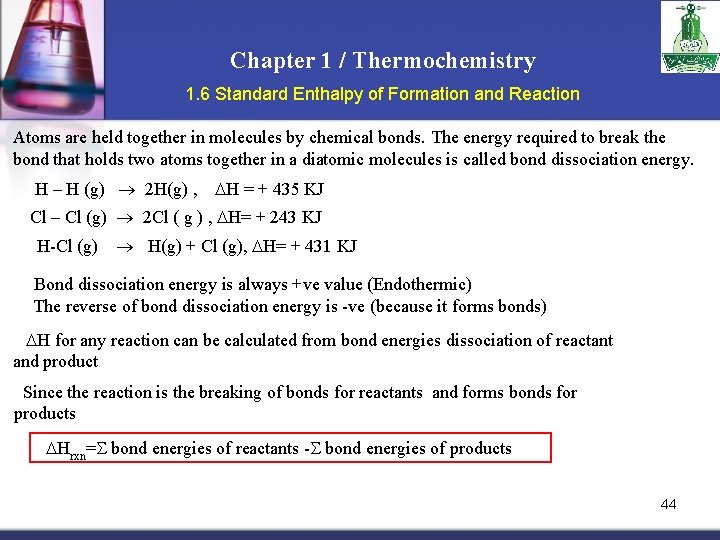
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Atoms are held together in molecules by chemical bonds. The energy required to break the bond that holds two atoms together in a diatomic molecules is called bond dissociation energy. H – H (g) 2 H(g) , H = + 435 KJ Cl – Cl (g) 2 Cl ( g ) , H= + 243 KJ H-Cl (g) H(g) + Cl (g), H= + 431 KJ Bond dissociation energy is always +ve value (Endothermic) The reverse of bond dissociation energy is -ve (because it forms bonds) H for any reaction can be calculated from bond energies dissociation of reactant and product Since the reaction is the breaking of bonds for reactants and forms bonds for products Hrxn= bond energies of reactants - bond energies of products 44

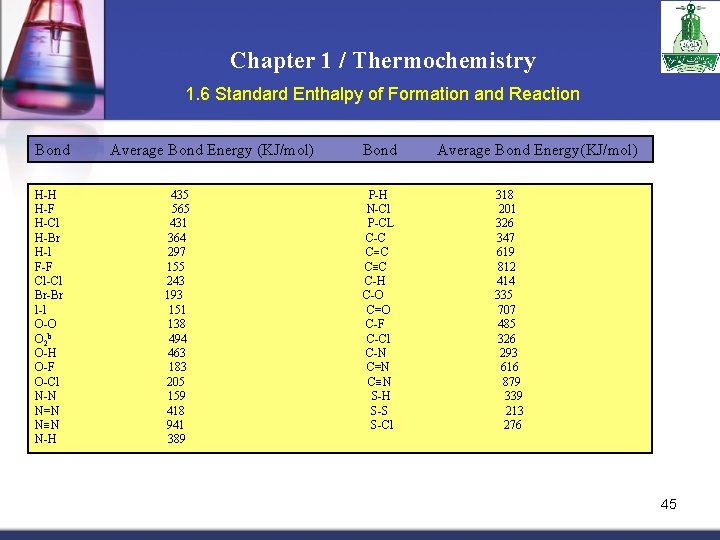
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Bond H-H H-F H-Cl H-Br H-l F-F Cl-Cl Br-Br l-l O-O O 2 b O-H O-F O-Cl N-N N=N N N N-H Average Bond Energy (KJ/mol) 435 565 431 364 297 155 243 193 151 138 494 463 183 205 159 418 941 389 Bond P-H N-Cl P-CL C-C C C C-H C-O C=O C-F C-Cl C-N C=N C N S-H S-S S-Cl Average Bond Energy(KJ/mol) 318 201 326 347 619 812 414 335 707 485 326 293 616 879 339 213 276 45

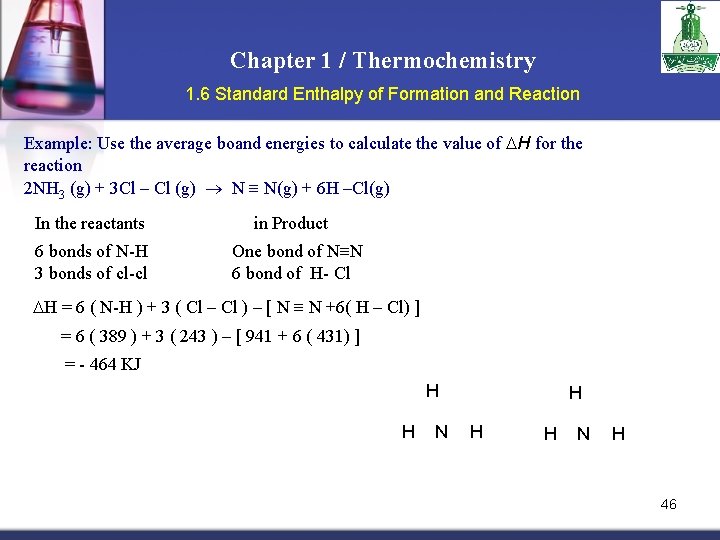
Chapter 1 / Thermochemistry 1. 6 Standard Enthalpy of Formation and Reaction Example: Use the average boand energies to calculate the value of H for the reaction 2 NH 3 (g) + 3 Cl – Cl (g) N N(g) + 6 H –Cl(g) In the reactants 6 bonds of N-H 3 bonds of cl-cl in Product One bond of N≡N 6 bond of H- Cl H = 6 ( N-H ) + 3 ( Cl – Cl ) – [ N N +6( H – Cl) ] = 6 ( 389 ) + 3 ( 243 ) – [ 941 + 6 ( 431) ] = - 464 KJ H H N H 46

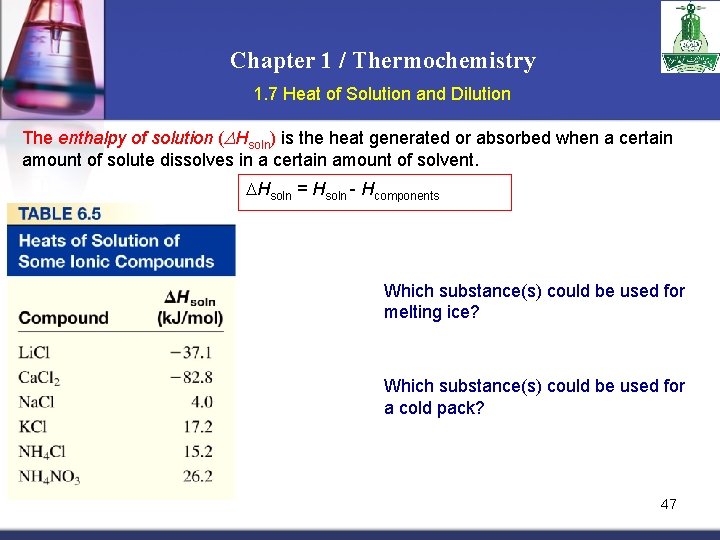
Chapter 1 / Thermochemistry 1. 7 Heat of Solution and Dilution The enthalpy of solution (DHsoln) is the heat generated or absorbed when a certain amount of solute dissolves in a certain amount of solvent. Hsoln = Hsoln - Hcomponents Which substance(s) could be used for melting ice? Which substance(s) could be used for a cold pack? 47

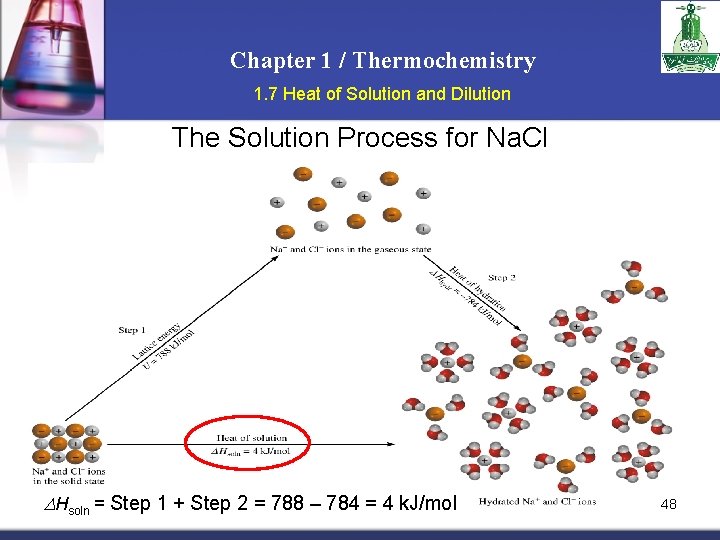
Chapter 1 / Thermochemistry 1. 7 Heat of Solution and Dilution The Solution Process for Na. Cl DHsoln = Step 1 + Step 2 = 788 – 784 = 4 k. J/mol 48

Chapter 1 / Thermochemistry 1. 4 Enthalpy of Chemical Reactions Thank you for listening 49