Chapter 5 Thermochemistry Part A Thermochemistry is the



- Slides: 39

Chapter 5 Thermochemistry Part A Thermochemistry is the study of energies given off by or absorbed by reactions Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved.

Law of Conservation of Energy Ability to do work or to transfer heat Kinetic Energy (KE) Energy of motion= ½mv 2 Potential Energy (PE) Stored energy, due to attractions & repulsions Chemical Energy Stored in chemical bonds Law of Conservation of Energy can neither be created nor destroyed. Can only be converted from one form to another Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 2

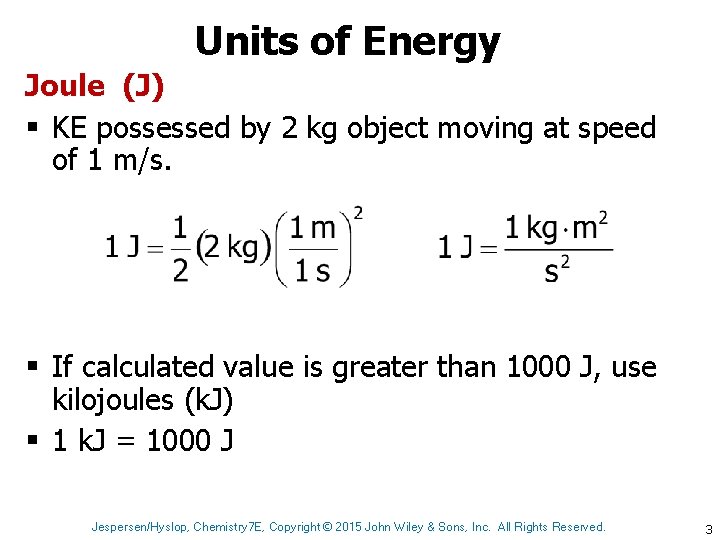
Units of Energy Joule (J) § KE possessed by 2 kg object moving at speed of 1 m/s. § If calculated value is greater than 1000 J, use kilojoules (k. J) § 1 k. J = 1000 J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 3

Units of Energy A calorie (cal) § Energy needed to raise the temperature of 1 g H 2 O by 1 °C § 1 cal = 4. 184 J (exactly) § 1 kcal = 1000 cal § 1 kcal = 4. 184 k. J A nutritional Calorie (Cal) § 1 Cal = 1000 cal = 1 kcal = 4. 184 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 4

Defining the System § What we are interested in studying § Reaction in beaker Surroundings § Everything else § Room in which reaction is run Boundary § Separation between system and surroundings Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 5

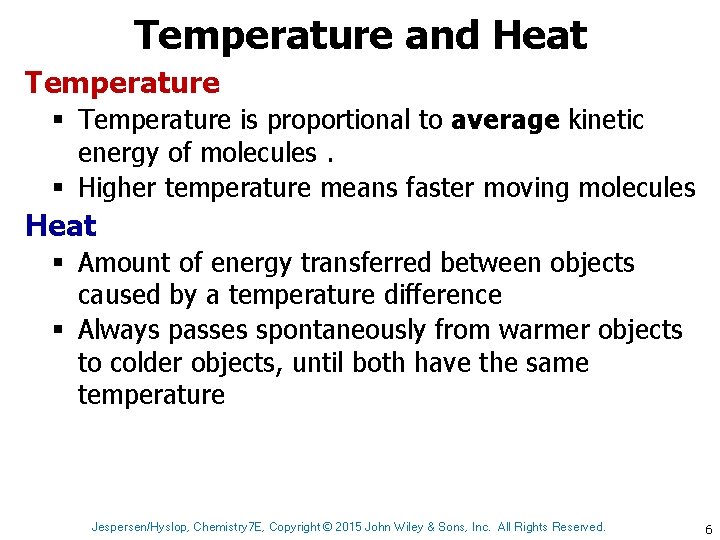
Temperature and Heat Temperature § Temperature is proportional to average kinetic energy of molecules. § Higher temperature means faster moving molecules Heat § Amount of energy transferred between objects caused by a temperature difference § Always passes spontaneously from warmer objects to colder objects, until both have the same temperature Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 6

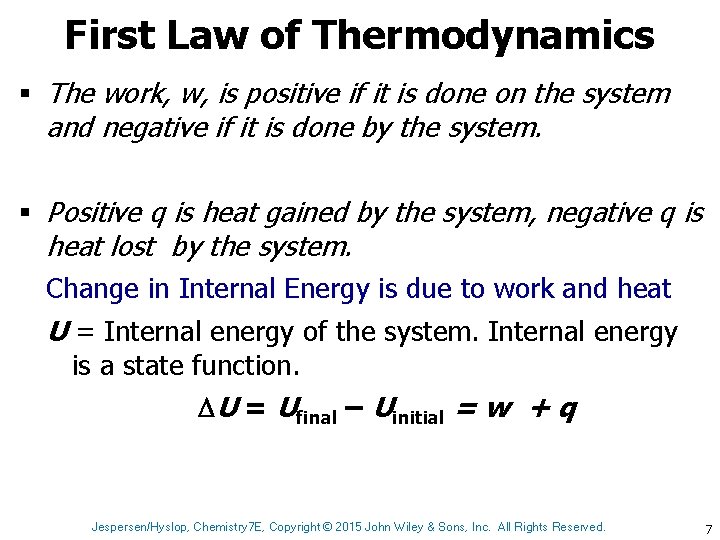
First Law of Thermodynamics § The work, w, is positive if it is done on the system and negative if it is done by the system. § Positive q is heat gained by the system, negative q is heat lost by the system. Change in Internal Energy is due to work and heat U = Internal energy of the system. Internal energy is a state function. U = Ufinal – Uinitial = w + q Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 7

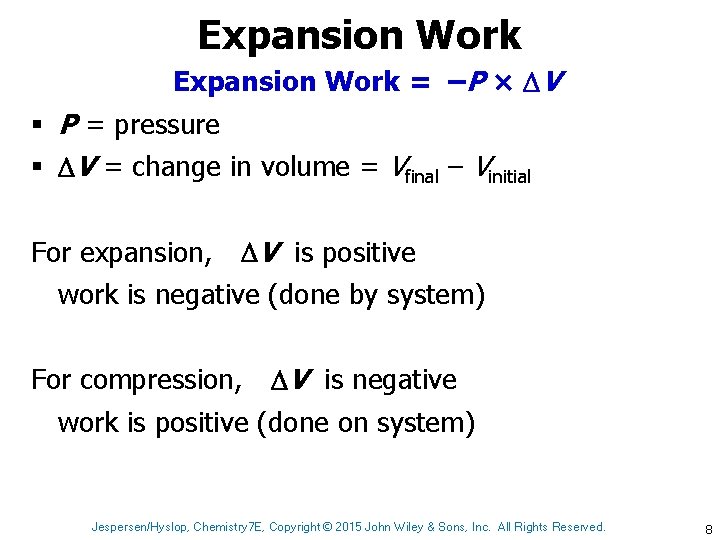
Expansion Work = –P × V § P = pressure § V = change in volume = Vfinal – Vinitial For expansion, V is positive work is negative (done by system) For compression, V is negative work is positive (done on system) Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 8

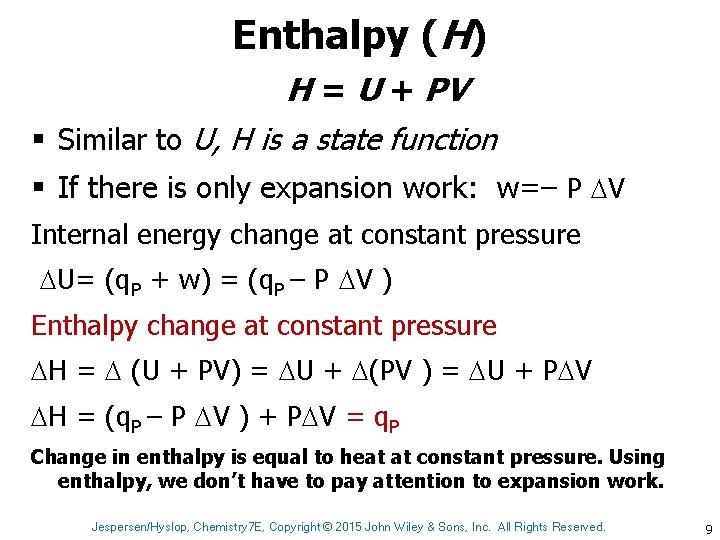
Enthalpy (H) H = U + PV § Similar to U, H is a state function § If there is only expansion work: w=– P V Internal energy change at constant pressure U= (q. P + w) = (q. P – P V ) Enthalpy change at constant pressure H = (U + PV) = U + (PV ) = U + P V H = (q. P – P V ) + P V = q. P Change in enthalpy is equal to heat at constant pressure. Using enthalpy, we don’t have to pay attention to expansion work. Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 9

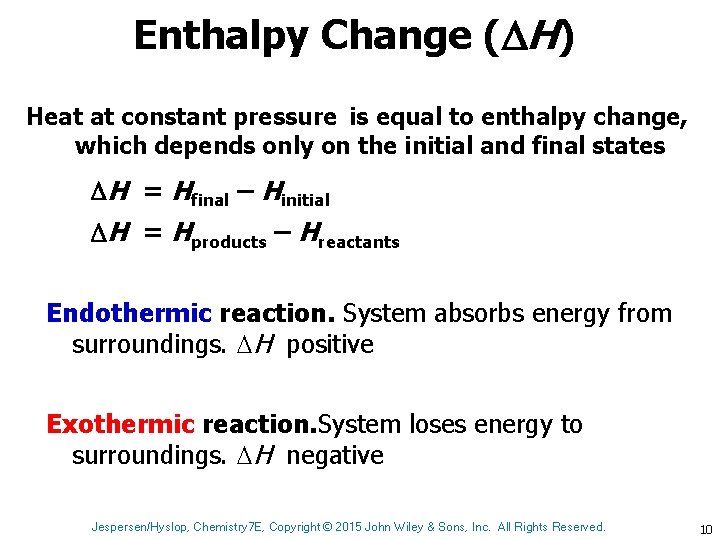
Enthalpy Change ( H) Heat at constant pressure is equal to enthalpy change, which depends only on the initial and final states H = Hfinal – Hinitial H = Hproducts – Hreactants Endothermic reaction. System absorbs energy from surroundings. H positive Exothermic reaction. System loses energy to surroundings. H negative Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 10

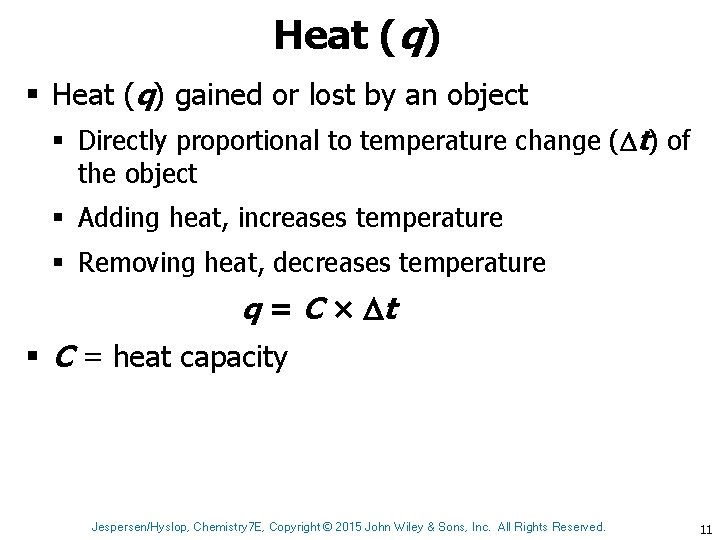
Heat (q) § Heat (q) gained or lost by an object § Directly proportional to temperature change ( t) of the object § Adding heat, increases temperature § Removing heat, decreases temperature q = C × t § C = heat capacity Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 11

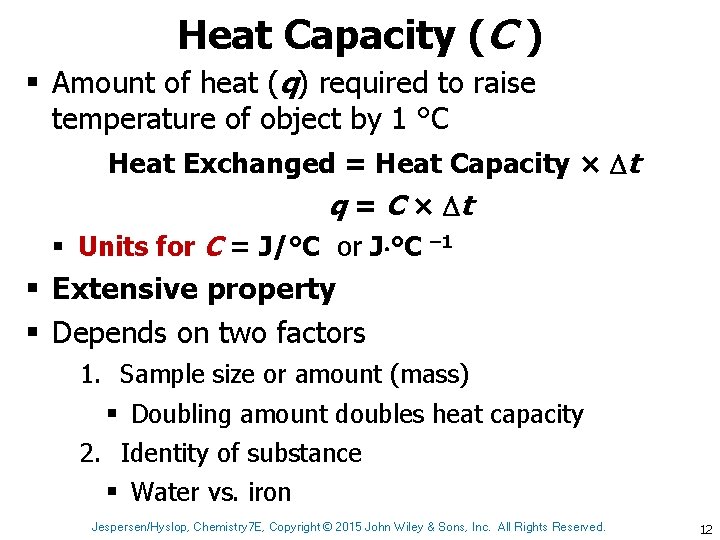
Heat Capacity (C ) § Amount of heat (q) required to raise temperature of object by 1 °C Heat Exchanged = Heat Capacity × t q = C × t § Units for C = J/°C or J °C – 1 § Extensive property § Depends on two factors 1. Sample size or amount (mass) § Doubling amount doubles heat capacity 2. Identity of substance § Water vs. iron Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 12

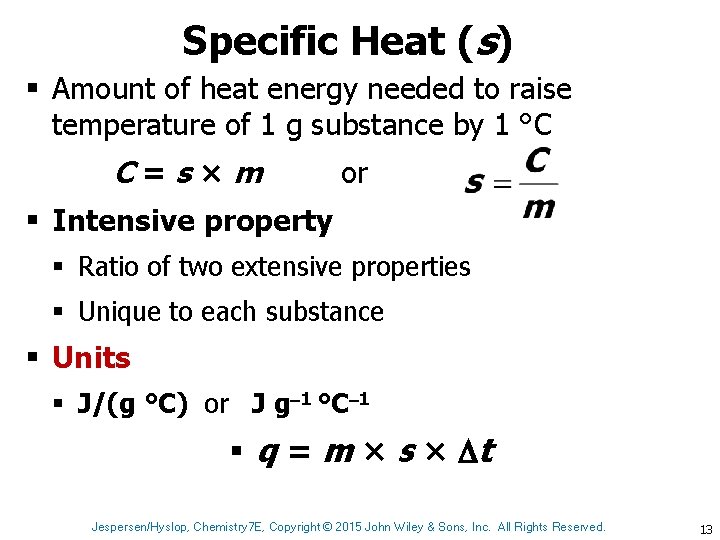
Specific Heat (s) § Amount of heat energy needed to raise temperature of 1 g substance by 1 °C C=s×m or § Intensive property § Ratio of two extensive properties § Unique to each substance § Units § J/(g °C) or J g 1 °C 1 § q = m × s × t Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 13

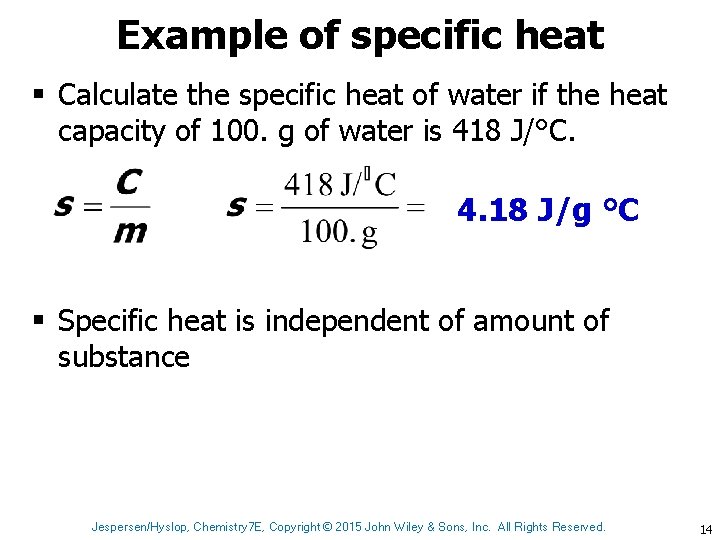
Example of specific heat § Calculate the specific heat of water if the heat capacity of 100. g of water is 418 J/°C. 4. 18 J/g °C § Specific heat is independent of amount of substance Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 14

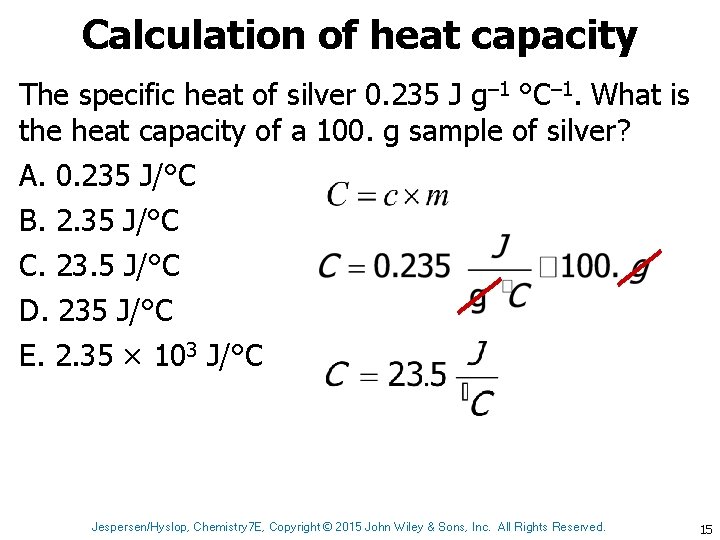
Calculation of heat capacity The specific heat of silver 0. 235 J g– 1 °C– 1. What is the heat capacity of a 100. g sample of silver? A. 0. 235 J/°C B. 2. 35 J/°C C. 23. 5 J/°C D. 235 J/°C E. 2. 35 × 103 J/°C Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 15

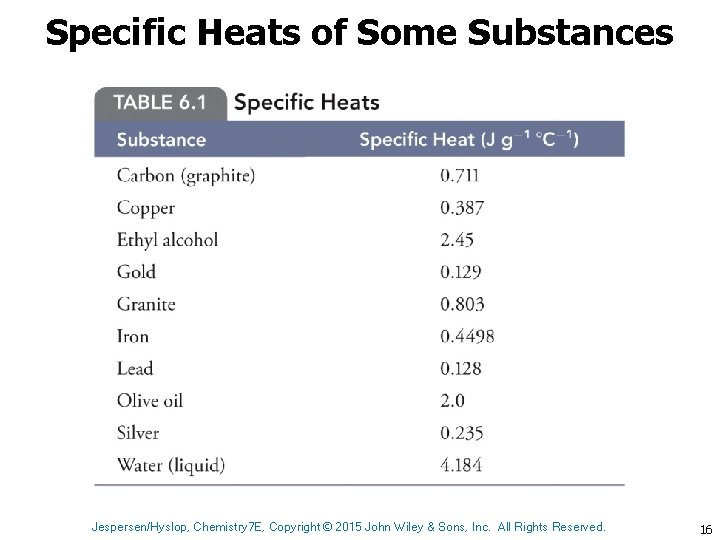
Specific Heats of Some Substances Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 16

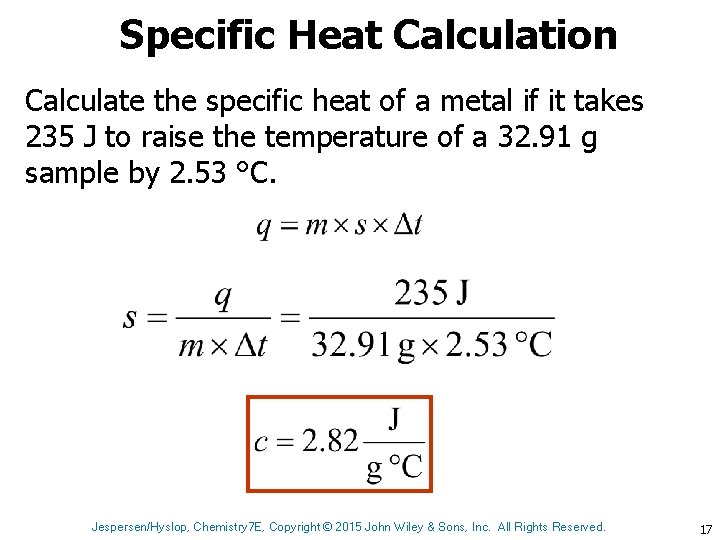
Specific Heat Calculation Calculate the specific heat of a metal if it takes 235 J to raise the temperature of a 32. 91 g sample by 2. 53 °C. Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 17

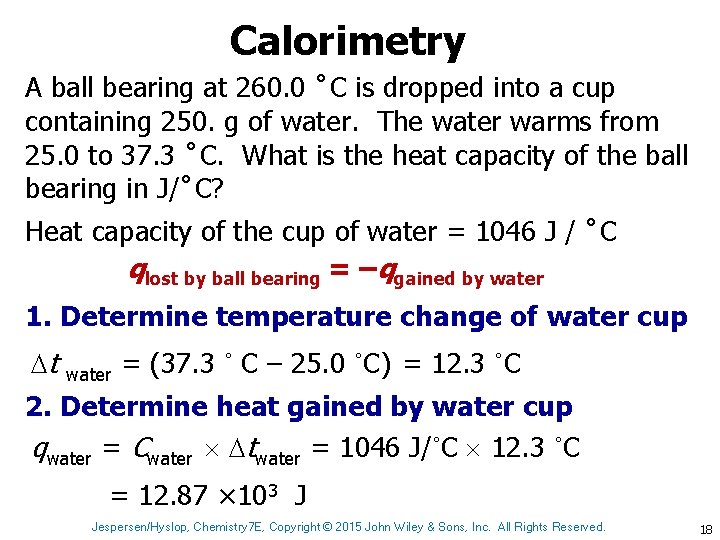
Calorimetry A ball bearing at 260. 0 ˚C is dropped into a cup containing 250. g of water. The water warms from 25. 0 to 37. 3 ˚C. What is the heat capacity of the ball bearing in J/˚C? Heat capacity of the cup of water = 1046 J / ˚C qlost by ball bearing = –qgained by water 1. Determine temperature change of water cup t water = (37. 3 ˚ C – 25. 0 ˚C) = 12. 3 ˚C 2. Determine heat gained by water cup qwater = Cwater twater = 1046 J/˚C 12. 3 ˚C = 12. 87 × 103 J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 18

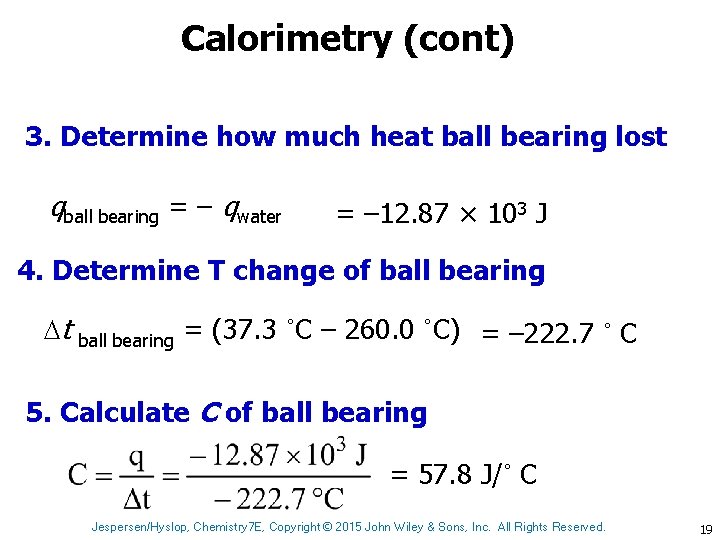
Calorimetry (cont) 3. Determine how much heat ball bearing lost qball bearing = – qwater = – 12. 87 × 103 J 4. Determine T change of ball bearing t ball bearing = (37. 3 ˚C – 260. 0 ˚C) = – 222. 7 ˚ C 5. Calculate C of ball bearing = 57. 8 J/˚ C Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 19

Heat of Reaction § Amount of heat absorbed or released in chemical reaction Calorimeter § Instrument used to measure temperature changes § Container of known heat capacity § Use results to calculate heat of reaction Calorimetry § Science of using calorimeter to determine heats of reaction Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 20

Heats of Reaction § Calorimeter design not standard § Depends on § Type of reaction § Precision desired § Usually measure heat of reaction under one of two sets of conditions § Constant volume, q. V § Closed, rigid container § Constant pressure, q. P § Open to atmosphere Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 21

Bomb Calorimeter Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 22

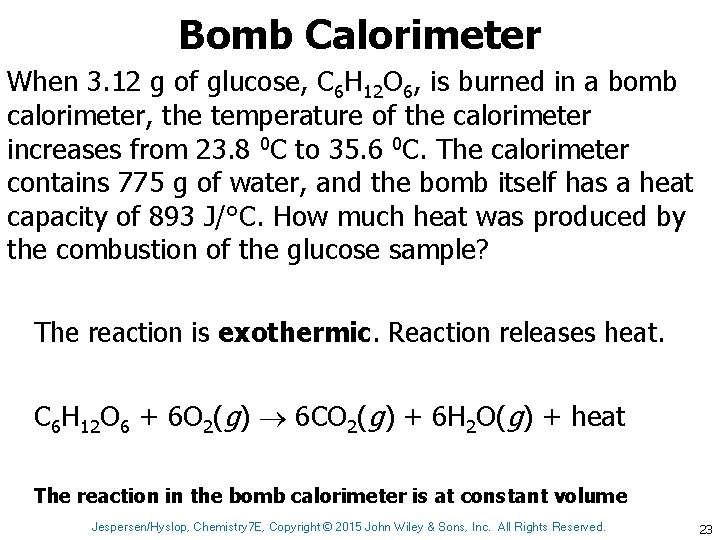
Bomb Calorimeter When 3. 12 g of glucose, C 6 H 12 O 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases from 23. 8 0 C to 35. 6 0 C. The calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 J/°C. How much heat was produced by the combustion of the glucose sample? The reaction is exothermic. Reaction releases heat. C 6 H 12 O 6 + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g) + heat The reaction in the bomb calorimeter is at constant volume Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 23

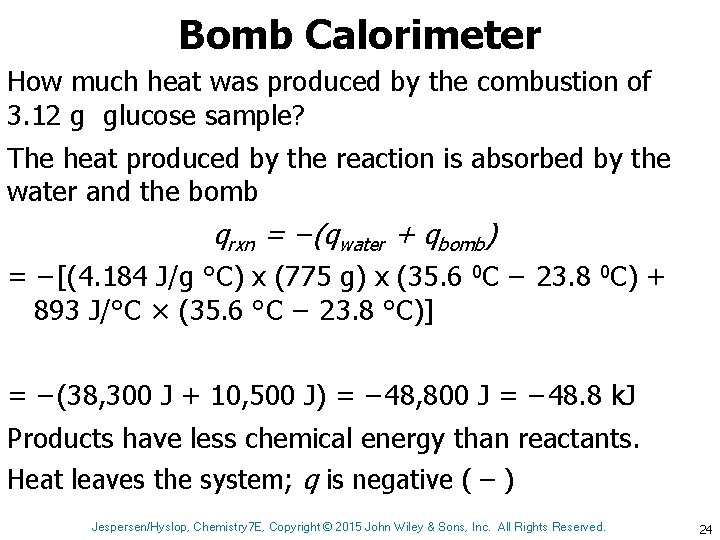
Bomb Calorimeter How much heat was produced by the combustion of 3. 12 g glucose sample? The heat produced by the reaction is absorbed by the water and the bomb qrxn = −(qwater + qbomb) = −[(4. 184 J/g °C) x (775 g) x (35. 6 0 C − 23. 8 0 C) + 893 J/°C × (35. 6 °C − 23. 8 °C)] = −(38, 300 J + 10, 500 J) = − 48, 800 J = − 48. 8 k. J Products have less chemical energy than reactants. Heat leaves the system; q is negative ( – ) Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 24

Endothermic Reaction § Reaction where products have more chemical energy than reactants § Reaction absorbs heat from surroundings § Heat added to system; q is positive (+) Example: Photosynthesis 6 CO 2(g) + 6 H 2 O(g) + solar energy C 6 H 12 O 6(s) + 6 O 2(g) Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 25

Enthalpy Changes in Chemical Reactions § Focus on systems § Endothermic § Reactants + heat products § Exothermic § Reactants products + heat § Want convenient way to calculate reaction enthalpies § Need way to tabulate enthalpies of reactions Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 26

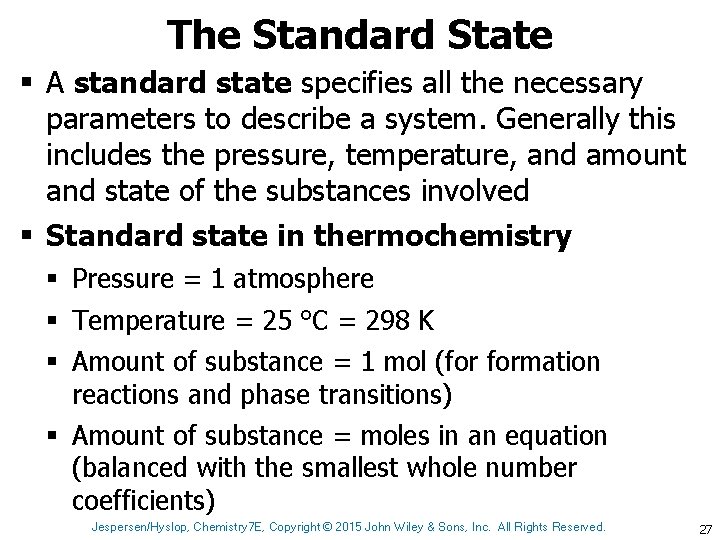
The Standard State § A standard state specifies all the necessary parameters to describe a system. Generally this includes the pressure, temperature, and amount and state of the substances involved § Standard state in thermochemistry § Pressure = 1 atmosphere § Temperature = 25 °C = 298 K § Amount of substance = 1 mol (for formation reactions and phase transitions) § Amount of substance = moles in an equation (balanced with the smallest whole number coefficients) Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 27

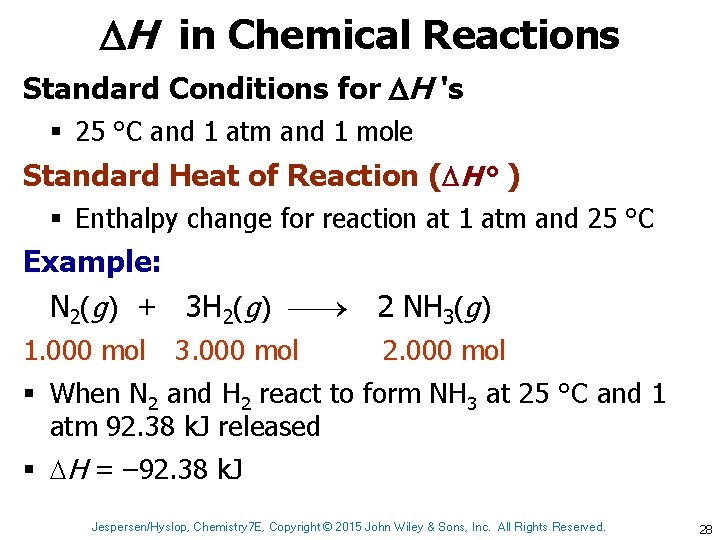
H in Chemical Reactions Standard Conditions for H 's § 25 °C and 1 atm and 1 mole Standard Heat of Reaction ( H ° ) § Enthalpy change for reaction at 1 atm and 25 °C Example: N 2(g) + 3 H 2(g) 2 NH 3(g) 1. 000 mol 3. 000 mol 2. 000 mol § When N 2 and H 2 react to form NH 3 at 25 °C and 1 atm 92. 38 k. J released § H = – 92. 38 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 28

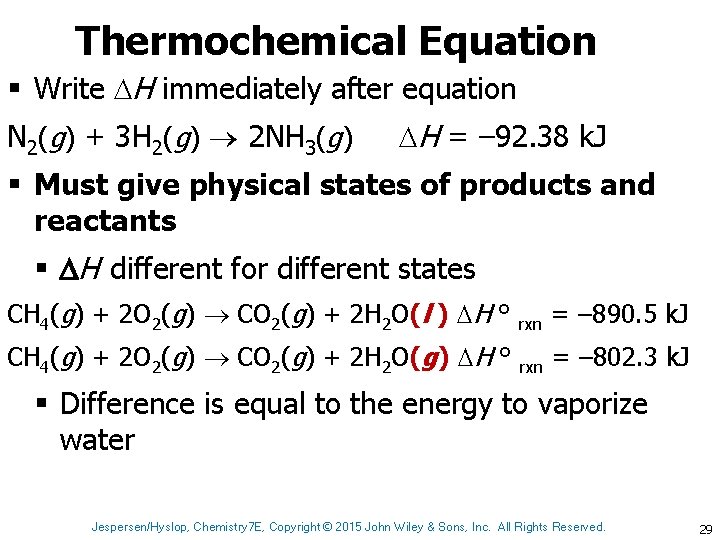
Thermochemical Equation § Write H immediately after equation N 2(g) + 3 H 2(g) 2 NH 3(g) H = – 92. 38 k. J § Must give physical states of products and reactants § H different for different states CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l ) H ° rxn = – 890. 5 k. J CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H ° rxn = – 802. 3 k. J § Difference is equal to the energy to vaporize water Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 29

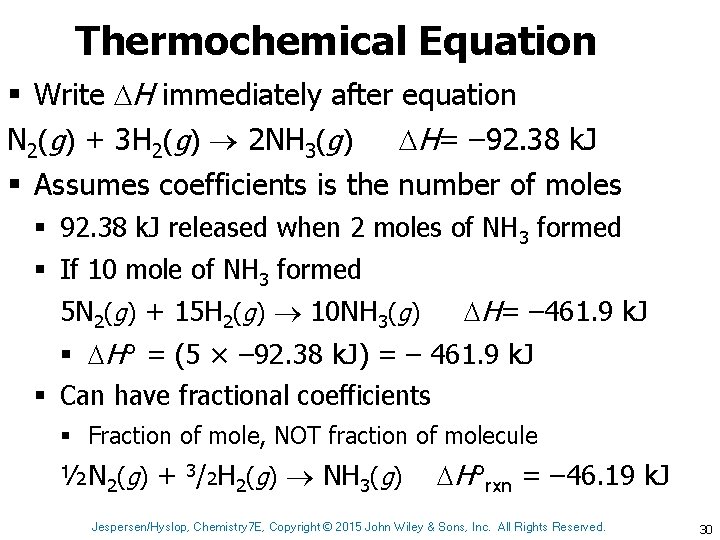
Thermochemical Equation § Write H immediately after equation N 2(g) + 3 H 2(g) 2 NH 3(g) H= – 92. 38 k. J § Assumes coefficients is the number of moles § 92. 38 k. J released when 2 moles of NH 3 formed § If 10 mole of NH 3 formed 5 N 2(g) + 15 H 2(g) 10 NH 3(g) H= – 461. 9 k. J § H° = (5 × – 92. 38 k. J) = – 461. 9 k. J § Can have fractional coefficients § Fraction of mole, NOT fraction of molecule ½N 2(g) + 3/2 H 2(g) NH 3(g) H°rxn = – 46. 19 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 30

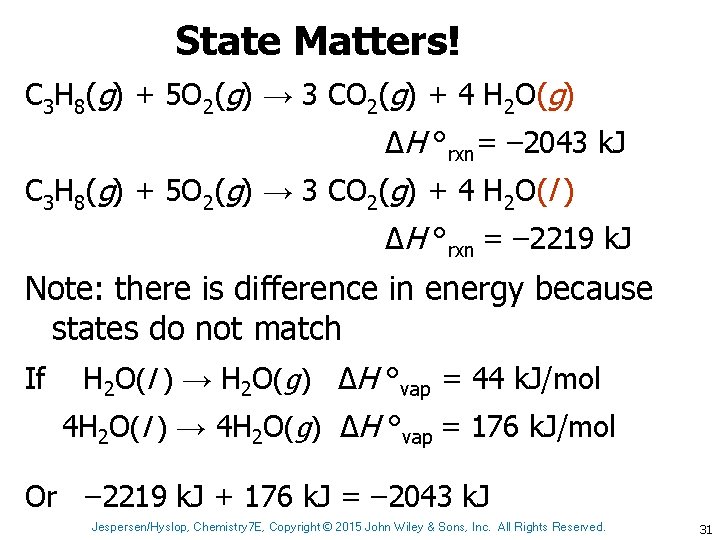
State Matters! C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) ΔH °rxn= – 2043 k. J C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l ) ΔH °rxn = – 2219 k. J Note: there is difference in energy because states do not match If H 2 O(l ) → H 2 O(g) ΔH °vap = 44 k. J/mol 4 H 2 O(l ) → 4 H 2 O(g) ΔH °vap = 176 k. J/mol Or – 2219 k. J + 176 k. J = – 2043 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 31

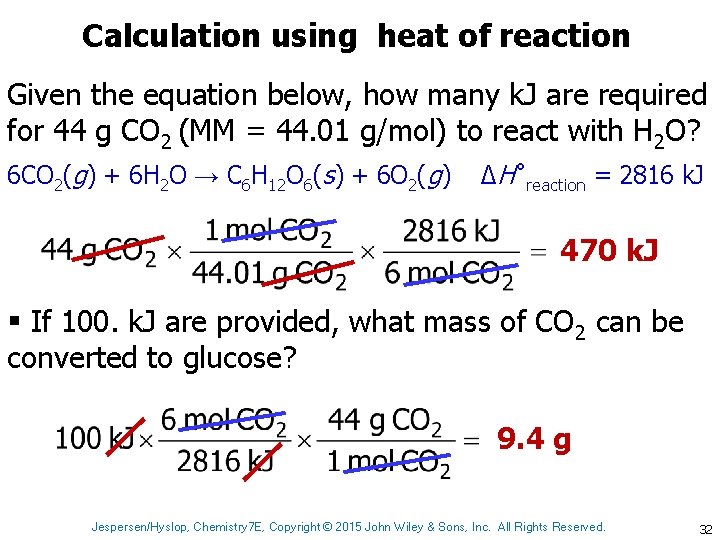
Calculation using heat of reaction Given the equation below, how many k. J are required for 44 g CO 2 (MM = 44. 01 g/mol) to react with H 2 O? 6 CO 2(g) + 6 H 2 O → C 6 H 12 O 6(s) + 6 O 2(g) ΔH˚reaction = 2816 k. J 470 k. J § If 100. k. J are provided, what mass of CO 2 can be converted to glucose? 9. 4 g Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 32

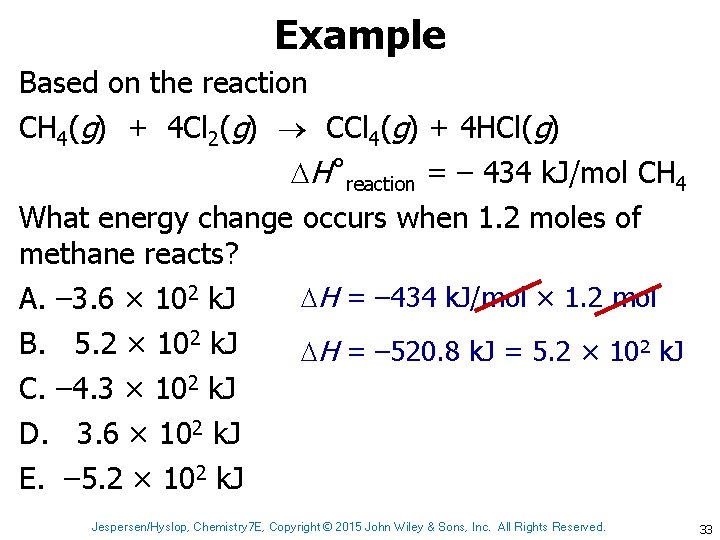
Example Based on the reaction CH 4(g) + 4 Cl 2(g) CCl 4(g) + 4 HCl(g) H˚reaction = – 434 k. J/mol CH 4 What energy change occurs when 1. 2 moles of methane reacts? H = – 434 k. J/mol × 1. 2 mol A. – 3. 6 × 102 k. J B. 5. 2 × 102 k. J H = – 520. 8 k. J = 5. 2 × 102 k. J C. – 4. 3 × 102 k. J D. 3. 6 × 102 k. J E. – 5. 2 × 102 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 33

Question Rxn. 1: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) ΔH °rxn= – 2043 k. J Rxn. 2: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l ) ΔH °rxn = – 2219 k. J Why does rxn. 2 release more heat than rxn. 1? A. It shouldn’t, and the H values should be equal B. More reactants must have been used in rxn. 2 C. In rxn. 1 some of the heat of the reaction is used up converting liquid water to gas, so less heat can be given off. D. Liquids always have lower temperatures than gases Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 34

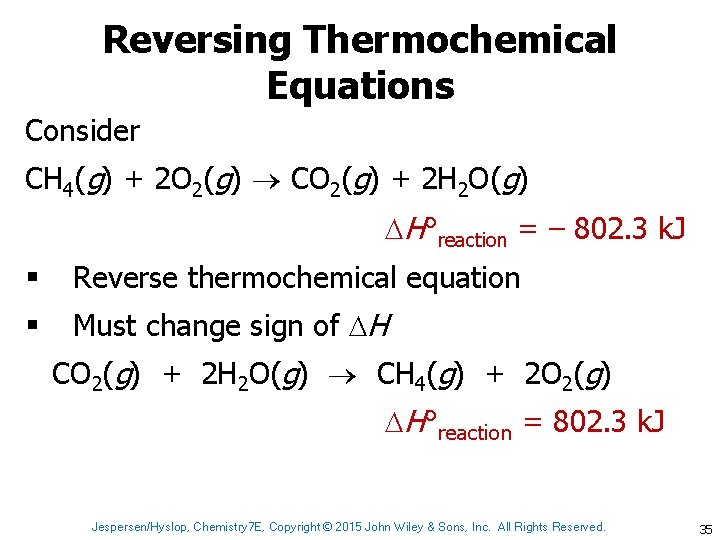
Reversing Thermochemical Equations Consider CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H°reaction = – 802. 3 k. J § Reverse thermochemical equation § Must change sign of H CO 2(g) + 2 H 2 O(g) CH 4(g) + 2 O 2(g) H°reaction = 802. 3 k. J Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 35

Reversing Thermochemical Equations Changes sign of H § Makes sense: § Get energy out when form products § Must put energy in to go back to reactants § Consequence of Law of Conservation of Energy § Like mathematical equation § If you know H ° for reaction, you also know H ° for the reverse Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 36

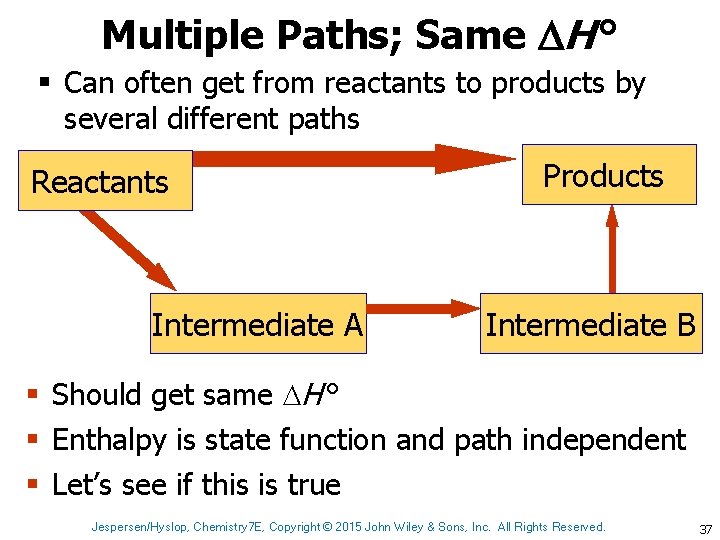
Multiple Paths; Same H ° § Can often get from reactants to products by several different paths Reactants Intermediate A Products Intermediate B § Should get same H ° § Enthalpy is state function and path independent § Let’s see if this is true Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 37

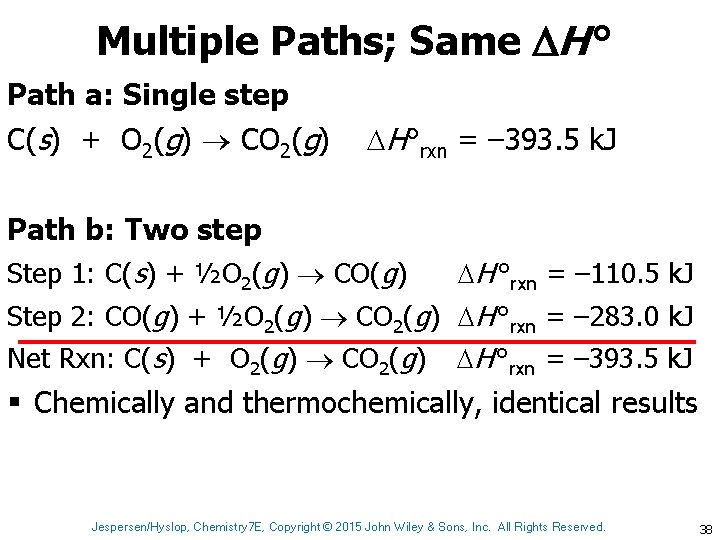
Multiple Paths; Same H ° Path a: Single step C(s) + O 2(g) CO 2(g) H°rxn = – 393. 5 k. J Path b: Two step Step 1: C(s) + ½O 2(g) CO(g) H °rxn = – 110. 5 k. J Step 2: CO(g) + ½O 2(g) CO 2(g) H °rxn = – 283. 0 k. J Net Rxn: C(s) + O 2(g) CO 2(g) H °rxn = – 393. 5 k. J § Chemically and thermochemically, identical results Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 38

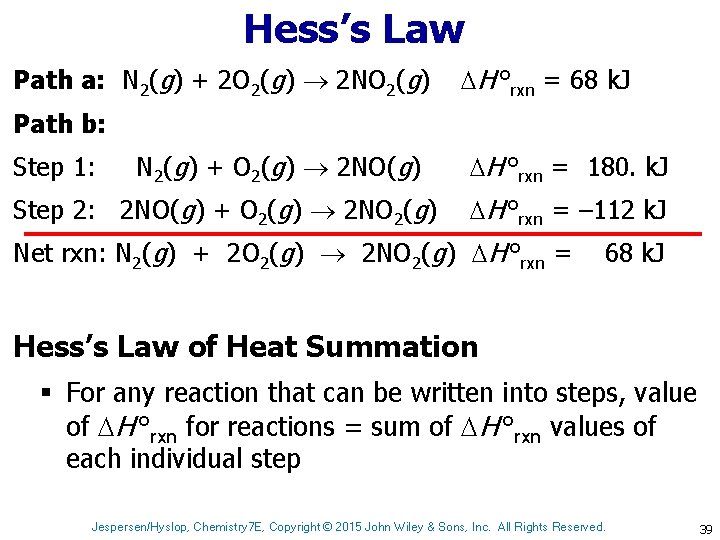
Hess’s Law Path a: N 2(g) + 2 O 2(g) 2 NO 2(g) H °rxn = 68 k. J Path b: Step 1: N 2(g) + O 2(g) 2 NO(g) Step 2: 2 NO(g) + O 2(g) 2 NO 2(g) H °rxn = 180. k. J H °rxn = – 112 k. J Net rxn: N 2(g) + 2 O 2(g) 2 NO 2(g) H °rxn = 68 k. J Hess’s Law of Heat Summation § For any reaction that can be written into steps, value of H °rxn for reactions = sum of H °rxn values of each individual step Jespersen/Hyslop, Chemistry 7 E, Copyright © 2015 John Wiley & Sons, Inc. All Rights Reserved. 39