MR HADDAD CHEMISTRY 101520 Thermochemistry THERMOCHEMISTRY Thermochemistry the




- Slides: 12

MR. HADDAD CHEMISTRY 10/15/20 Thermochemistry

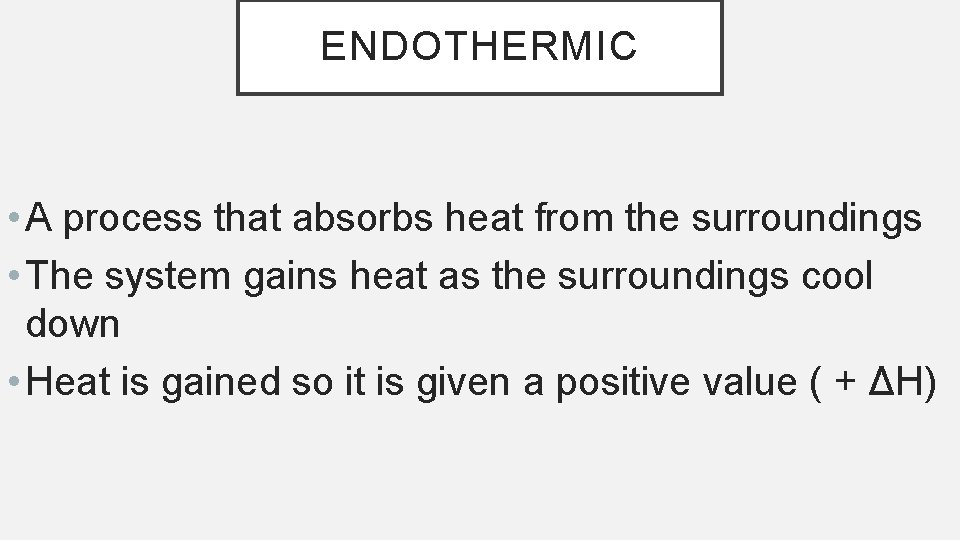
THERMOCHEMISTRY • Thermochemistry = the study of the heat changes that occur during chemical reactions and physical changes of state. • It involves absorbing or releasing heat. • Heat (q) = energy that transfers from one object into another because of a temperature difference between them. • Heat always flows from a warmer object to a cooler object.

ENERGY • Energy = The capacity to do work or supply heat • Chemical Potential Energy = The energy stored in chemicals because of their bonds • Different substances store different amounts of energy • Law of Conservation of Energy = States that in any chemical or physical process, energy is neither created nor destroyed. • All the energy involved in a process can be used for work, stored energy, or heat.

ENTHALPY (ΔH) • The heat content of a system • ΔH can be positive or negative • A system is the part of the universe which you focus your attention. • The surroundings include everything else in the universe.

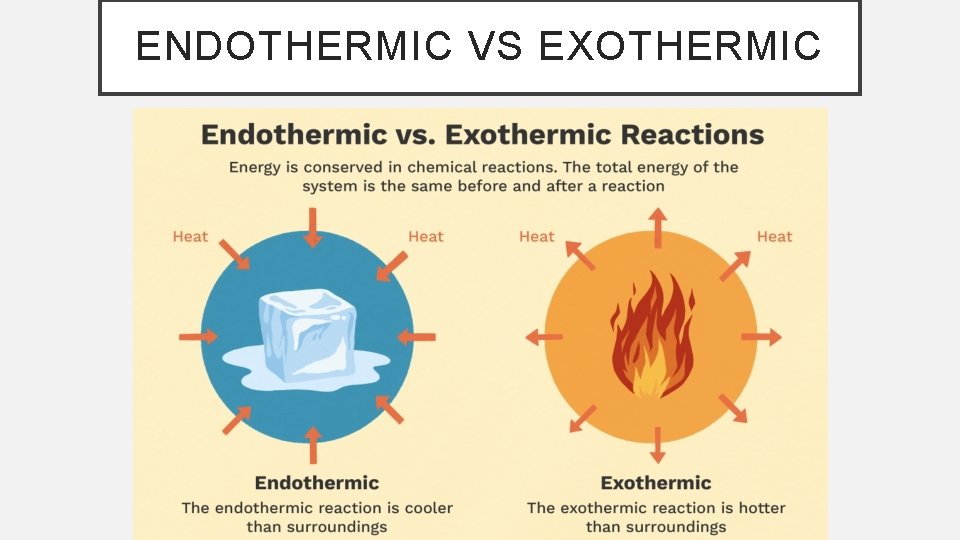
EXOTHERMIC • A process that loses heat to the surroundings • The system loses heat as the surroundings heat up • Heat is lost so it is given a negative value ( - ΔH)

ENDOTHERMIC • A process that absorbs heat from the surroundings • The system gains heat as the surroundings cool down • Heat is gained so it is given a positive value ( + ΔH)

ENDOTHERMIC VS EXOTHERMIC

UNITS FOR MEASURING HEAT FLOW • Heat flow is measured in two ways; the calorie and the joule • A calorie (cal) is defined as the heat needed to raise the temperature of 1 gram of pure water 1 degree Celsius • 1 Calorie = 1 Kilocalorie = 1000 • 1 Joule = 0. 2390 Cal • 4. 184 Joule = 1 Cal

CALORIMETRY • Is the accurate and precise measurement of the heat change for chemical and physical processes • To measure heat change or phase changes, reactions must be carried out in insulated containers • Calorimeters – devices used to measure the amount of heat absorbed or released during a chemical or physical process

THERMOCHEMICAL EQUATIONS EXAMPLES • Endothermic Example • Reactants + HEAT Products • 2 Na. HCO 3(s) + HEAT Na 2 CO 3(s) + H 2 O(g) + CO 2(g) • Exothermic Example • Reactants Products + HEAT • Ca. O(s) + H 2 O(L) Ca(OH)2(s) + HEAT

PHASE CHANGES • SOLID LIQUID GAS • GAS LIQUID SOLID • Endothermic Reaction • Exothermic Reaction • Heat is being added to the system • Heat is being removed from the system

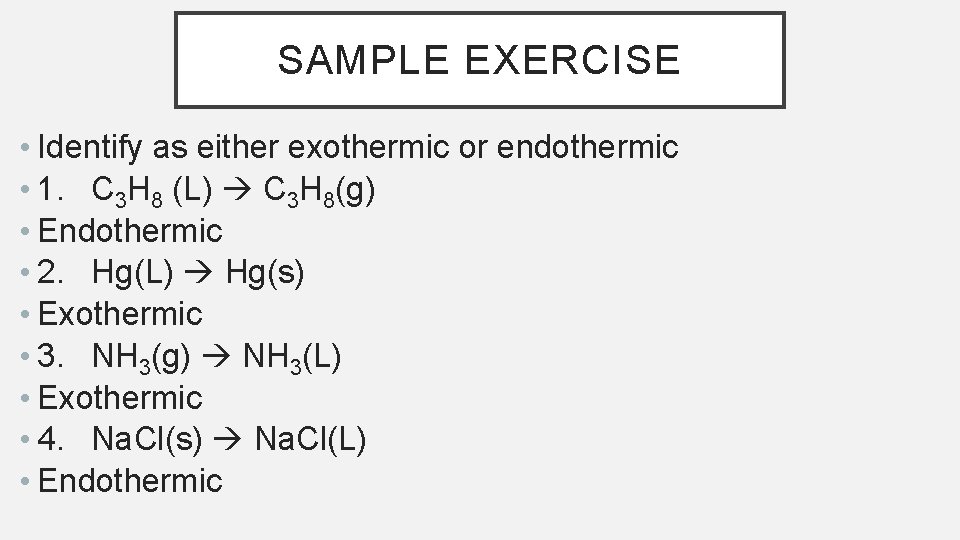
SAMPLE EXERCISE • Identify as either exothermic or endothermic • 1. C 3 H 8 (L) C 3 H 8(g) • Endothermic • 2. Hg(L) Hg(s) • Exothermic • 3. NH 3(g) NH 3(L) • Exothermic • 4. Na. Cl(s) Na. Cl(L) • Endothermic