Chapter 17 Thermochemistry Thermochemistry The study of energy



- Slides: 13

Chapter 17 Thermochemistry

Thermochemistry The study of energy changes that occur during chemical reactions and changes in state.

Thermochemistry Energy: the ability to do work or to supply heat Work is the force applied to move an object. ENERGY (Joule or calorie) = HEAT (J or cal) 1 cal = 4. 18 J Chemical Potential Energy: stored energy in chemicals

Heat vs. Temp. Heat and temperature are concepts that are often confused

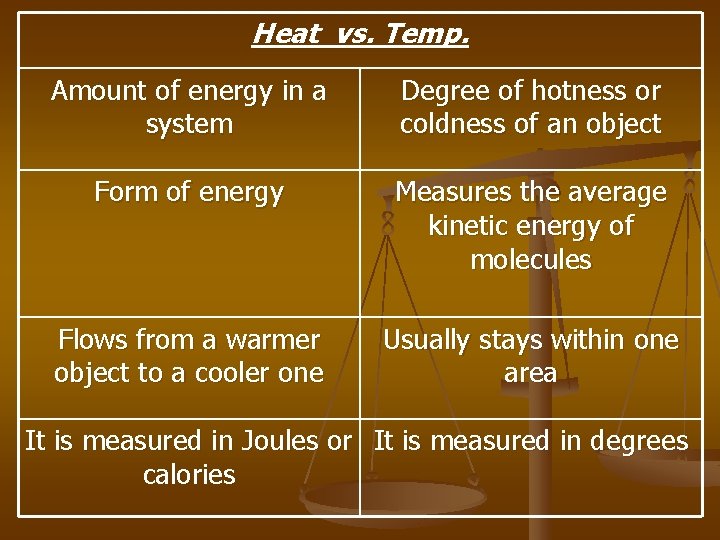
Heat vs. Temp. Amount of energy in a system Degree of hotness or coldness of an object Form of energy Measures the average kinetic energy of molecules Flows from a warmer object to a cooler one Usually stays within one area It is measured in Joules or It is measured in degrees calories

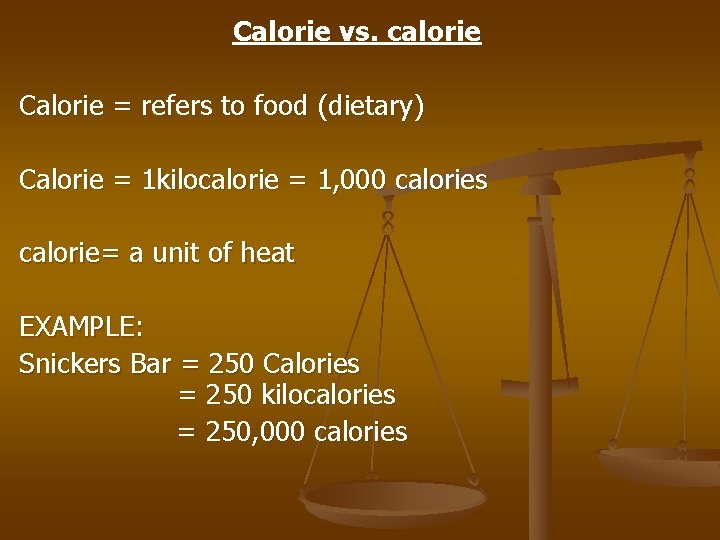
Calorie vs. calorie Calorie = refers to food (dietary) Calorie = 1 kilocalorie = 1, 000 calories calorie= a unit of heat EXAMPLE: Snickers Bar = 250 Calories = 250 kilocalories = 250, 000 calories

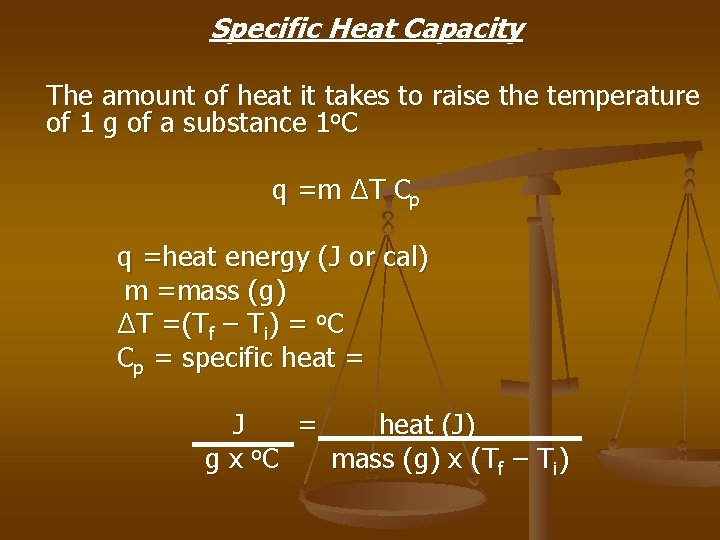
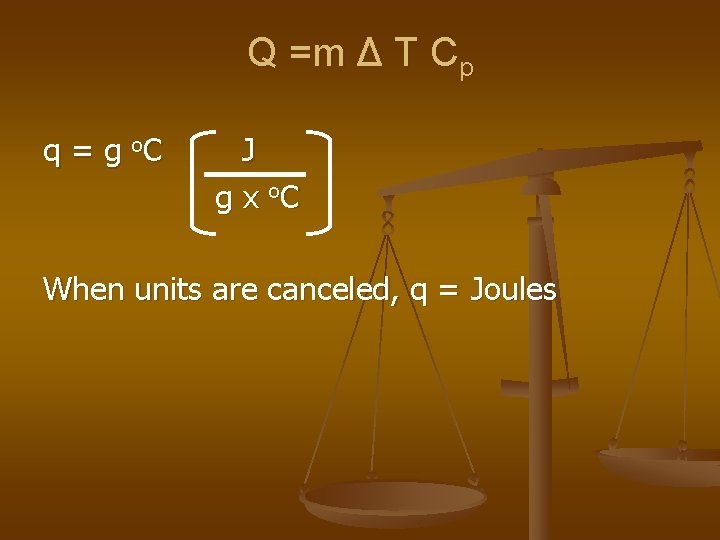
Specific Heat Capacity The amount of heat it takes to raise the temperature of 1 g of a substance 1 o. C q =m ΔT Cp q =heat energy (J or cal) m =mass (g) ΔT =(Tf – Ti) = o. C Cp = specific heat = J = heat (J) g x o. C mass (g) x (Tf – Ti)

Q =m Δ T Cp q = g o. C J g x o. C When units are canceled, q = Joules

Specific Heat Capacity The amount of heat it takes to raise the temperature of 1 g of a substance 1 o. C For example: WATER VS. METAL -Higher the specific heat: the less fluctuation of heat and therefore the less fluctuation of the temp. of the object. Water Iron 4. 18 J/(g x o. C) 0. 46 J/(g x o. C) High Low

Exothermic vs. Endothermic Exothermic: a process in which heat is released to the surrounding - The system loses heat as the surroundings heat up - q has a negative value because the system is losing heat Endothermic: a process in which the system gains heat as the surroundings cool down - Heat flows into a system - q has a positive value because the system is gaining heat

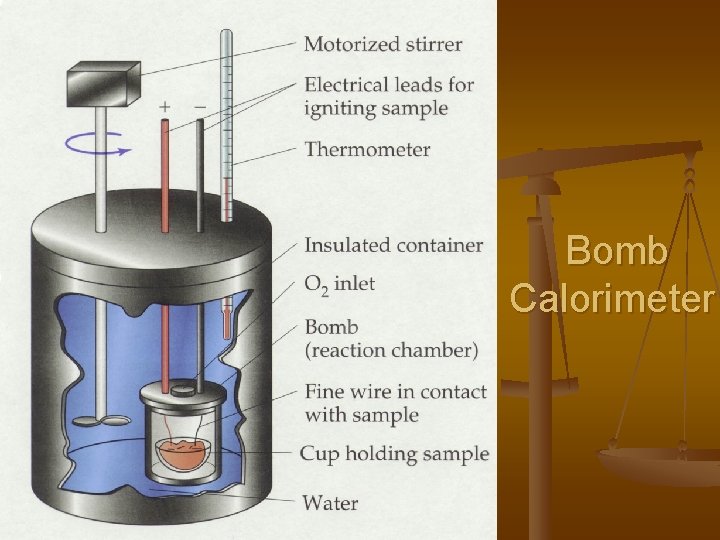
Calorimetry To measure specific heat capacity a bomb calorimeter is used. Calorimetry is based on the principle: Law of Conservation of Energy

Bomb Calorimeter

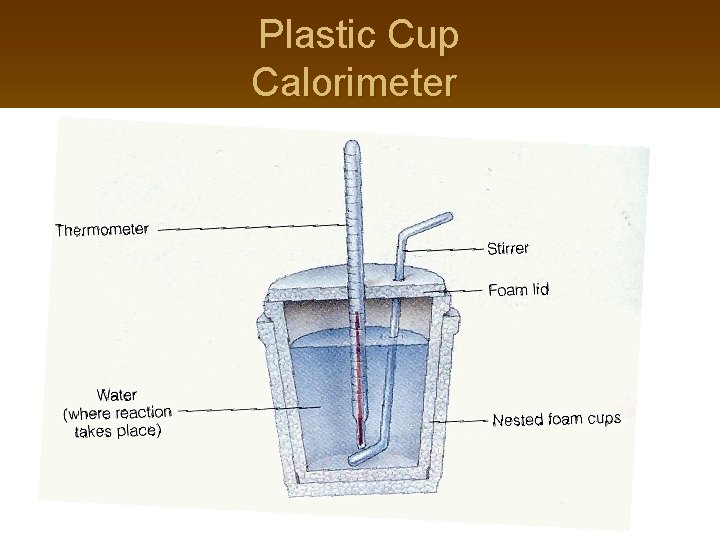
Plastic Cup Calorimeter
 Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of *
Thermochemistry is the study of * Study of energy transformations
Study of energy transformations Ilmu kimia yang mempelajari tentang panas/suhu disebut
Ilmu kimia yang mempelajari tentang panas/suhu disebut Thermochemistry is study of
Thermochemistry is study of Q = m x cp x delta t
Q = m x cp x delta t Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry