THERMOCHEMISTRY Thermochemistry is the study of the motion

- Slides: 13

THERMOCHEMISTRY Thermochemistry is the study of the motion of heat energy as it is transferred from the system to the surrounding or from the surrounding to the system. The transfer of heat could be due to a physical change or a chemical change.

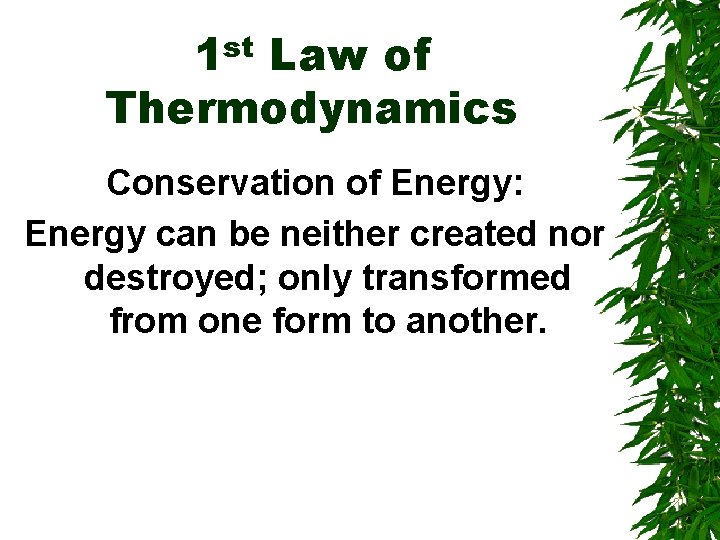
1 st Law of Thermodynamics Conservation of Energy: Energy can be neither created nor destroyed; only transformed from one form to another.

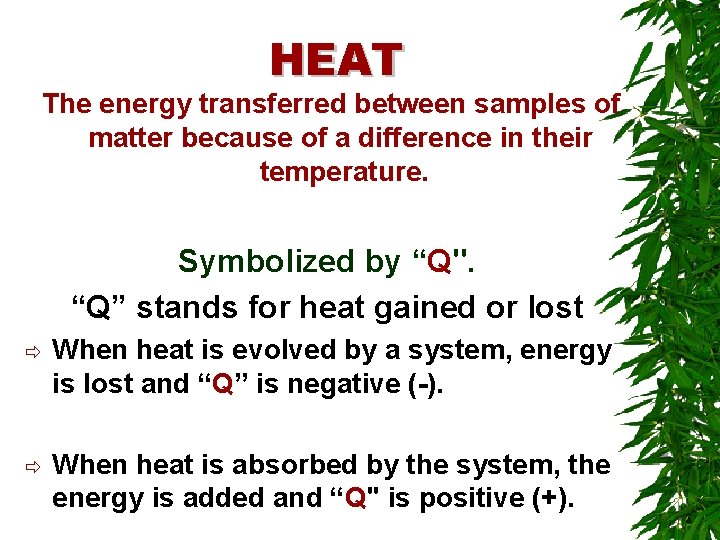
HEAT The energy transferred between samples of matter because of a difference in their temperature. Symbolized by “Q". “Q” stands for heat gained or lost ð When heat is evolved by a system, energy is lost and “Q” is negative (-). ð When heat is absorbed by the system, the energy is added and “Q" is positive (+).

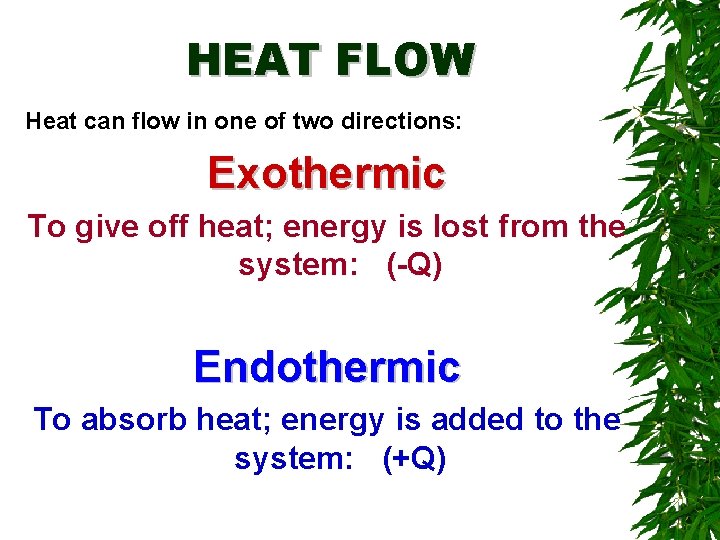
HEAT FLOW Heat can flow in one of two directions: Exothermic To give off heat; energy is lost from the system: (-Q) Endothermic To absorb heat; energy is added to the system: (+Q)

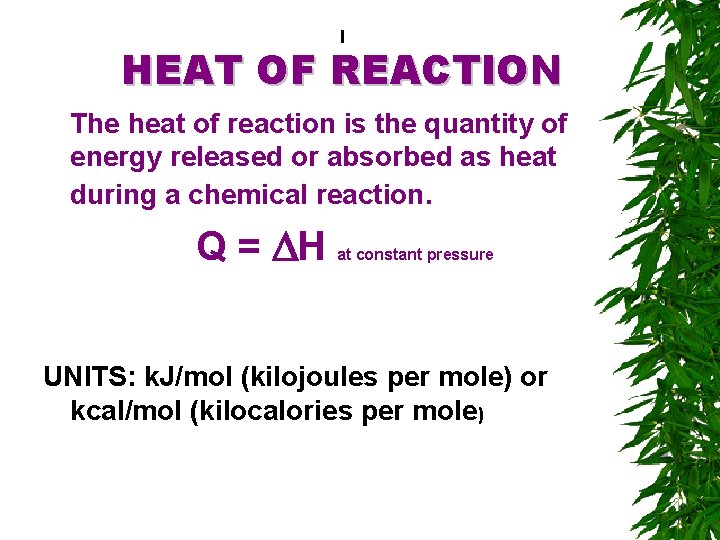
I HEAT OF REACTION The heat of reaction is the quantity of energy released or absorbed as heat during a chemical reaction. Q = DH at constant pressure UNITS: k. J/mol (kilojoules per mole) or kcal/mol (kilocalories per mole)

HEAT CAPACITY & SPECIFIC HEAT CAPACITY: The quantity of heat needed to raise the temperature of a substance one degree Celsius (or one Kelvin). Extensive property SPECIFIC HEAT: The quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin). Intensive property

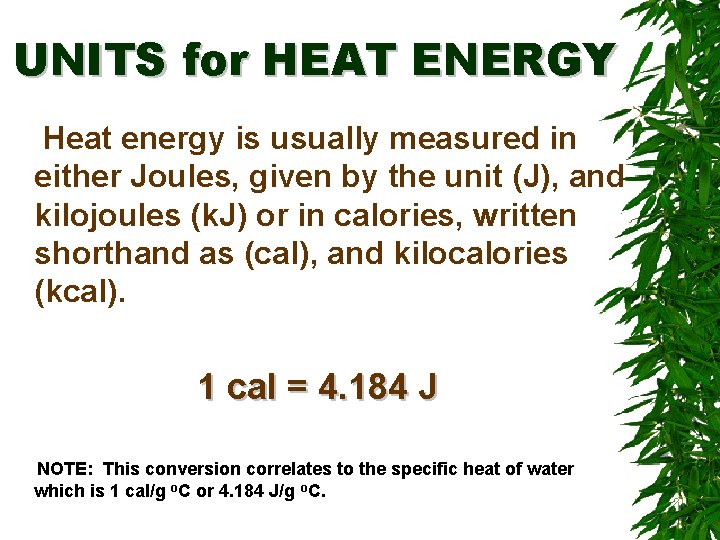
UNITS for HEAT ENERGY Heat energy is usually measured in either Joules, given by the unit (J), and kilojoules (k. J) or in calories, written shorthand as (cal), and kilocalories (kcal). 1 cal = 4. 184 J NOTE: This conversion correlates to the specific heat of water which is 1 cal/g o. C or 4. 184 J/g o. C.

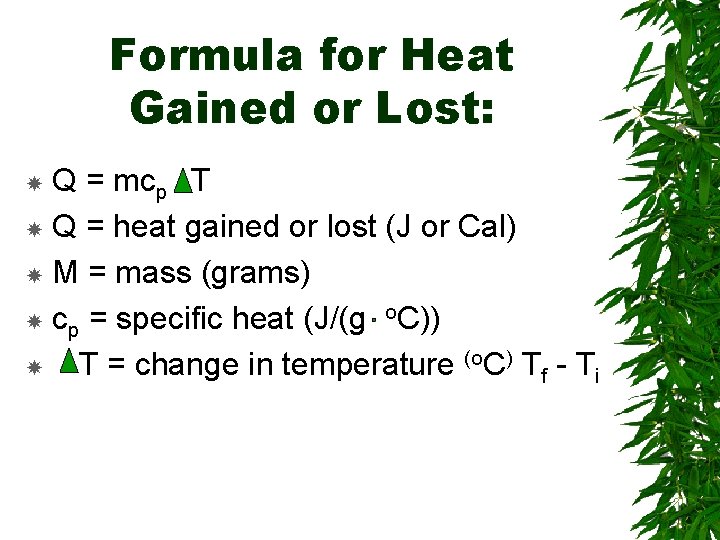
Formula for Heat Gained or Lost: Q = mcp T Q = heat gained or lost (J or Cal) M = mass (grams) cp = specific heat (J/(g o. C)) T = change in temperature (o. C) Tf - Ti

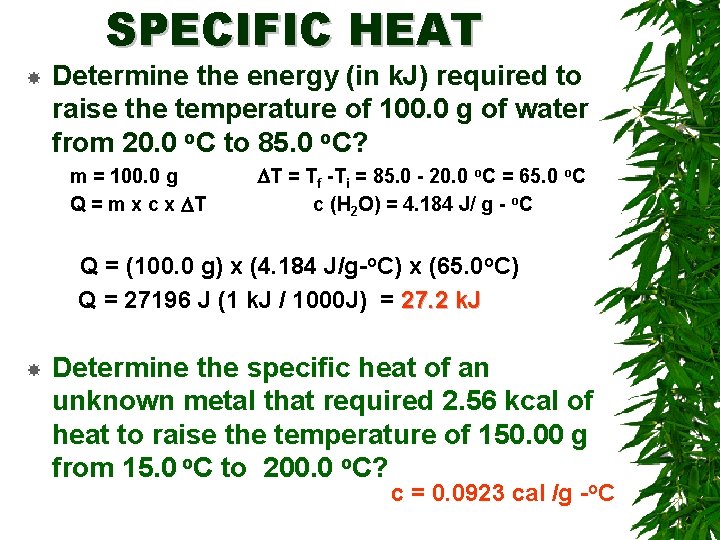
SPECIFIC HEAT Determine the energy (in k. J) required to raise the temperature of 100. 0 g of water from 20. 0 o. C to 85. 0 o. C? m = 100. 0 g Q = m x c x DT DT = Tf -Ti = 85. 0 - 20. 0 o. C = 65. 0 o. C c (H 2 O) = 4. 184 J/ g - o. C Q = (100. 0 g) x (4. 184 J/g-o. C) x (65. 0 o. C) Q = 27196 J (1 k. J / 1000 J) = 27. 2 k. J Determine the specific heat of an unknown metal that required 2. 56 kcal of heat to raise the temperature of 150. 00 g from 15. 0 o. C to 200. 0 o. C? c = 0. 0923 cal /g -o. C

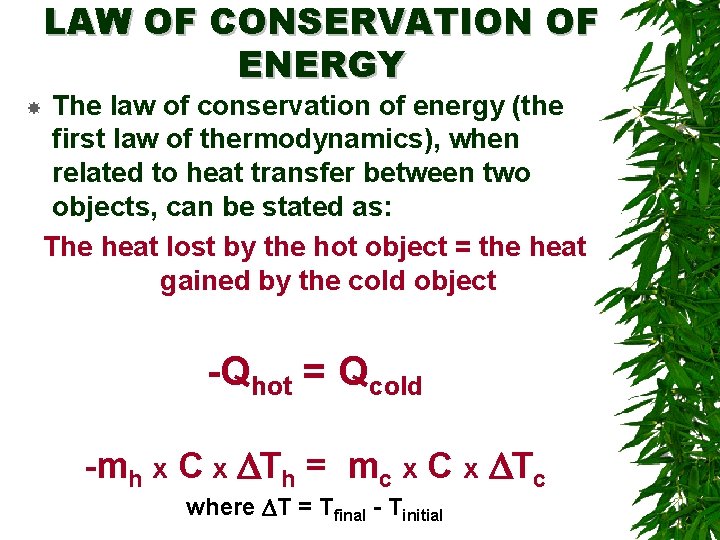
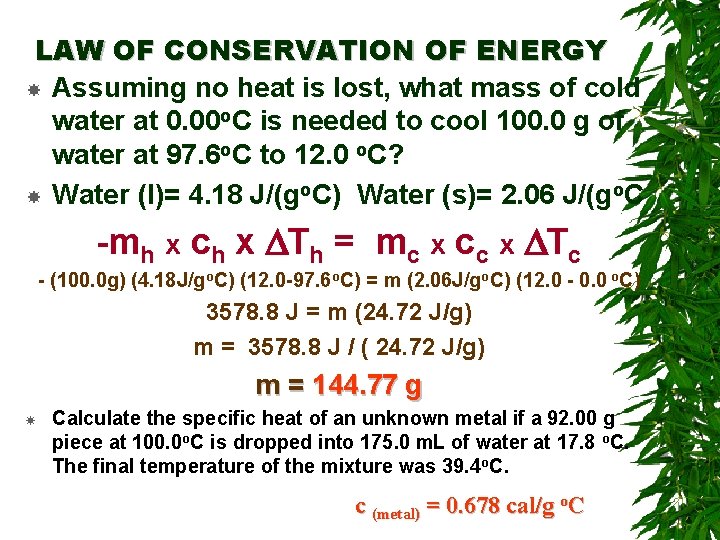
LAW OF CONSERVATION OF ENERGY The law of conservation of energy (the first law of thermodynamics), when related to heat transfer between two objects, can be stated as: The heat lost by the hot object = the heat gained by the cold object -Qhot = Qcold -mh x C x DTh = mc x C x DTc where DT = Tfinal - Tinitial

LAW OF CONSERVATION OF ENERGY Assuming no heat is lost, what mass of cold water at 0. 00 o. C is needed to cool 100. 0 g of water at 97. 6 o. C to 12. 0 o. C? Water (l)= 4. 18 J/(go. C) Water (s)= 2. 06 J/(go. C -mh x ch x DTh = mc x cc x DTc - (100. 0 g) (4. 18 J/go. C) (12. 0 -97. 6 o. C) = m (2. 06 J/go. C) (12. 0 - 0. 0 o. C) 3578. 8 J = m (24. 72 J/g) m = 3578. 8 J / ( 24. 72 J/g) m = 144. 77 g Calculate the specific heat of an unknown metal if a 92. 00 g piece at 100. 0 o. C is dropped into 175. 0 m. L of water at 17. 8 o. C. The final temperature of the mixture was 39. 4 o. C. c (metal) = 0. 678 cal/g o. C

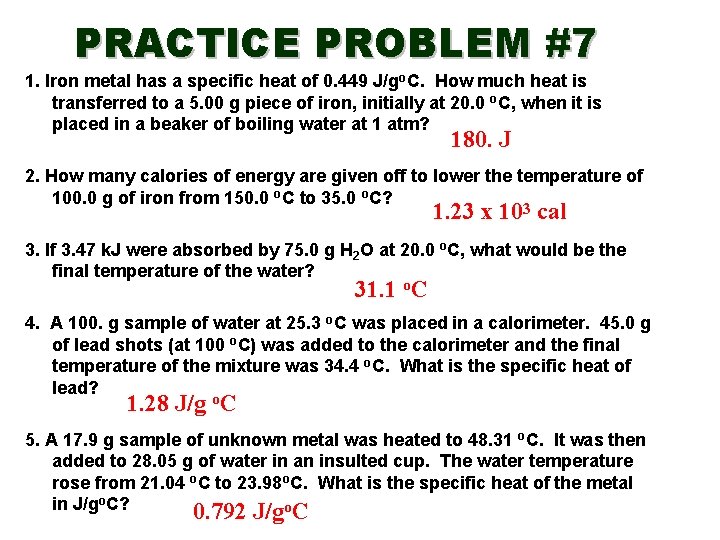
PRACTICE PROBLEM #7 1. Iron metal has a specific heat of 0. 449 J/go. C. How much heat is transferred to a 5. 00 g piece of iron, initially at 20. 0 o. C, when it is placed in a beaker of boiling water at 1 atm? 180. J 2. How many calories of energy are given off to lower the temperature of 100. 0 g of iron from 150. 0 o. C to 35. 0 o. C? 1. 23 x 103 cal 3. If 3. 47 k. J were absorbed by 75. 0 g H 2 O at 20. 0 o. C, what would be the final temperature of the water? 31. 1 o. C 4. A 100. g sample of water at 25. 3 o. C was placed in a calorimeter. 45. 0 g of lead shots (at 100 o. C) was added to the calorimeter and the final temperature of the mixture was 34. 4 o. C. What is the specific heat of lead? 1. 28 J/g o. C 5. A 17. 9 g sample of unknown metal was heated to 48. 31 o. C. It was then added to 28. 05 g of water in an insulted cup. The water temperature rose from 21. 04 o. C to 23. 98 o. C. What is the specific heat of the metal in J/go. C? 0. 792 J/go. C

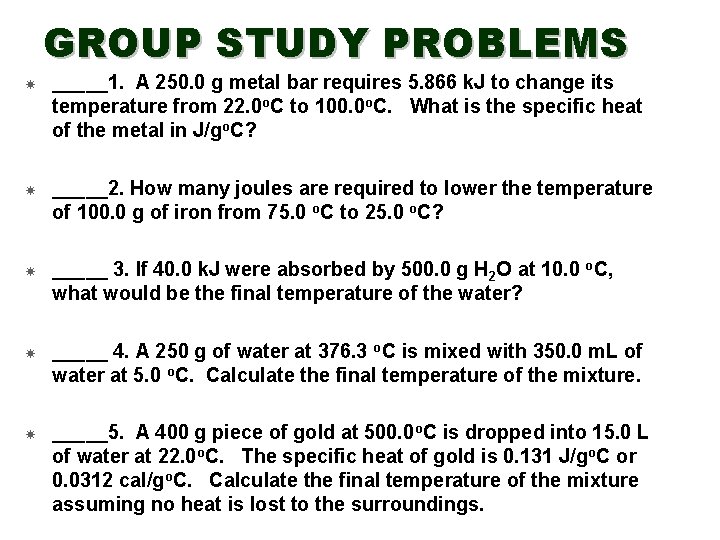
GROUP STUDY PROBLEMS _____1. A 250. 0 g metal bar requires 5. 866 k. J to change its temperature from 22. 0 o. C to 100. 0 o. C. What is the specific heat of the metal in J/go. C? _____2. How many joules are required to lower the temperature of 100. 0 g of iron from 75. 0 o. C to 25. 0 o. C? _____ 3. If 40. 0 k. J were absorbed by 500. 0 g H 2 O at 10. 0 o. C, what would be the final temperature of the water? _____ 4. A 250 g of water at 376. 3 o. C is mixed with 350. 0 m. L of water at 5. 0 o. C. Calculate the final temperature of the mixture. _____5. A 400 g piece of gold at 500. 0 o. C is dropped into 15. 0 L of water at 22. 0 o. C. The specific heat of gold is 0. 131 J/go. C or 0. 0312 cal/go. C. Calculate the final temperature of the mixture assuming no heat is lost to the surroundings.
 Distinguish between motion study and time study
Distinguish between motion study and time study Time and motion study example ppt
Time and motion study example ppt Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is a study of
Thermochemistry is a study of Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of
Thermochemistry is the study of Termokimia adalah cabang ilmu kimia yang mempelajari…
Termokimia adalah cabang ilmu kimia yang mempelajari… Thermochemistry is the study of
Thermochemistry is the study of Q = m x cp x ∆t
Q = m x cp x ∆t Passive rom vs active rom
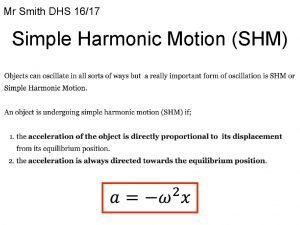
Passive rom vs active rom Periodic motion formula
Periodic motion formula An object in motion stays in motion
An object in motion stays in motion Chapter 2 motion section 1 describing motion answer key
Chapter 2 motion section 1 describing motion answer key