Thermochemistry AP Chemistry Mr G Thermochemistry The study






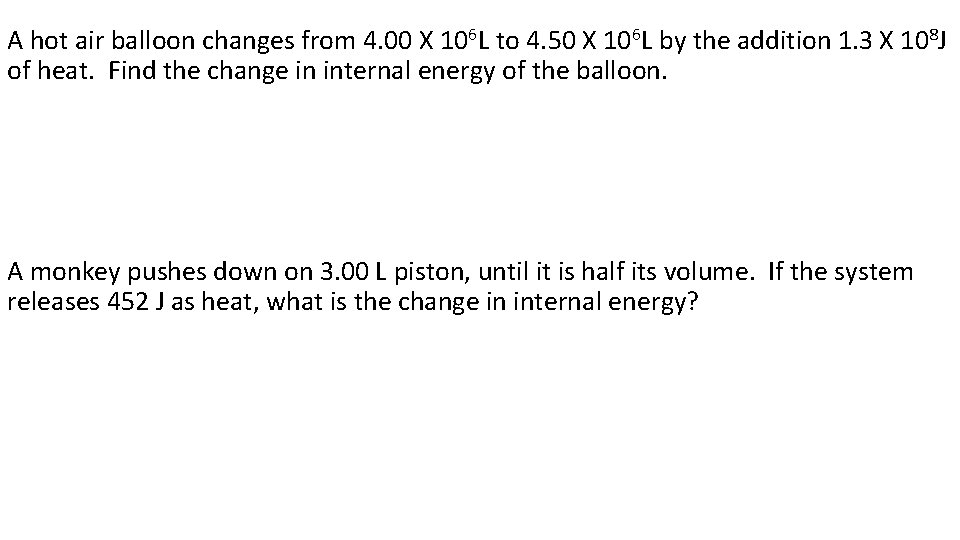











- Slides: 26

Thermochemistry AP Chemistry Mr. G

Thermochemistry- The study of Energy changes in matter. Energy- the capacity to do work or produce heat. 1 st Law of Thermodynamics- All the energy in the universe is constant. It cannot be created nor destroyed. It simply changes forms. Energy comes in many forms: Kinetic(translational) KE = 1/2 m. V 2, Rotational KE = 1/2 Iw 2, In a gas KE = 3/2 n. RT, etc. Potential (U) Energy: Positional PE = mgh, Spring U = ½ Kx 2, etc.

In Thermochemistry: • System(Sys. )- the part of the Universe on which you are focusing in on. (Usually the reaction) • Surroundings(Surr. )-the rest of the Universe. (container, room, air, oceans, mars, etc. ) • Universe(Univ. )-the system(Sys) + the surroundings(Surr. ) We can consider the system as a box: Surroundings System

Energy can enter or leave the system. It must do by work or heat. DE = work x heat Surroundings System

A gas expands and does 45 J of work. It also releases 15 J of heat. Find the change in internal energy. A gas is compressed and gains 78 J of work, while absorbing 88 J heat. A reaction does 63 J of work, while absorbing 27 J or heat. Find DE: Convert to e. V(1 e. V = 1. 60 x 10 -19 J) Convert to ergs(1 J = 1 x 107 erg)

Work-the amount of energy needed to push something a certain distance. • Work = Force x distance • In Chemistry, you have to find the amount of work associated with a gas in a piston. • (isobaric process- pressure is constant)

Finding work of a gas w/ DV (Adiabatic, q=0) • A gas in a piston is compressed from 882 ml to 645 ml against a constant 1. 25 atm. How much work is done & by who? • A gas expands from 22. 4 L to 35. 7 L against a constant 1. 55 atm. How much work is done? • A gas expands by 1. 6 liters, while W= - 472 J. What is the external pressure?
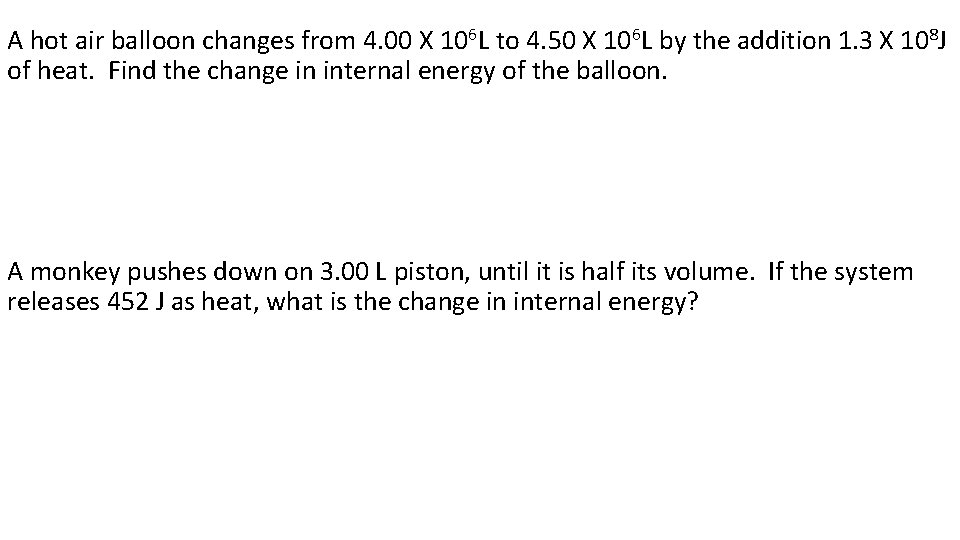
A hot air balloon changes from 4. 00 X 106 L to 4. 50 X 106 L by the addition 1. 3 X 108 J of heat. Find the change in internal energy of the balloon. A monkey pushes down on 3. 00 L piston, until it is half its volume. If the system releases 452 J as heat, what is the change in internal energy?

• AP Chem. Thermodynamics HW: • Write out each question. Box your answers. Work must be shown for credit. This is an INDIVIDUAL, NOT GROUP assignment. • p. 285, #'s: 4, 6 a, 26, 30, 32, 34, 38, 44, 46, 52, 56, 60, 72, 74, 76, 78, 82 • Due on test day.

Diabatic process- heat(q) is exchanged between system and surr. (q = 0) • Diabatic(when heat is exchanged between system and surroundings) processes can occur with or without a temperature change. • W/ temp. change: • q = m. Cp. DT • q = heat(Joules) m = mass Cp = specific heat (J/g mol) DT = Tf – Ti Specific heat- the amount of heat needed to raise the temp. of 1 gram(or mole) of subst. by 1 C o. How much heat is released when 25. 0 grams of water cool from 89 o. C to 35 o. C? (Cp. H 2 O = 4. 184 J/g. Co)

• Iron has a specific heat of 0. 450 J/g. Co. What mass of iron releases 122 J of heat when cooled from 50. 0 o. C to 47. 2 o. C? • How much heat is absorbed by 150. grams H 2 O when as it is warmed from 15. 0 o. C to 32. 5 o. C?

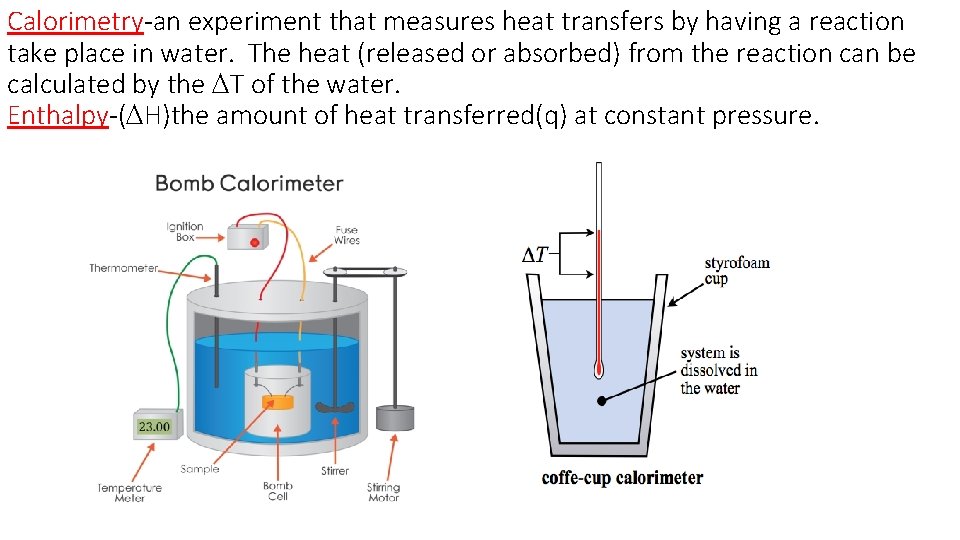
Calorimetry-an experiment that measures heat transfers by having a reaction take place in water. The heat (released or absorbed) from the reaction can be calculated by the DT of the water. Enthalpy-(DH)the amount of heat transferred(q) at constant pressure.

• The combustion of 3. 2 grams of ethane in a calorimeter raises the temperature of 800. 0 grams of water from 25. 0 o. C to 49. 8 o. C. • a) write the balanced molecular equation for the reaction: • b) How much heat(q) was released by the reaction? • c) What is the molar enthalpy of the reaction? • d) write the balanced molecular equation including heat: • e) How much heat would burning 42. 5 g ethane release?

• In a coffee cup calorimeter, 100. ml of 1. 00 M HCl is neutralized by 100. ml of 1. 00 M Na. OH. Both solutions were at 24. 2 o. C but warmed to 31. 3 o. C when the reaction was over. a) Write the balanced equation for the rxn: b) How much heat was released by the rxn? c) Find the molar enthalpy of neutralization for the rxn: d) Include the heat into the balanced equation: e) How much heat would be released by neutralizing 25. 0 grams Na. OH?

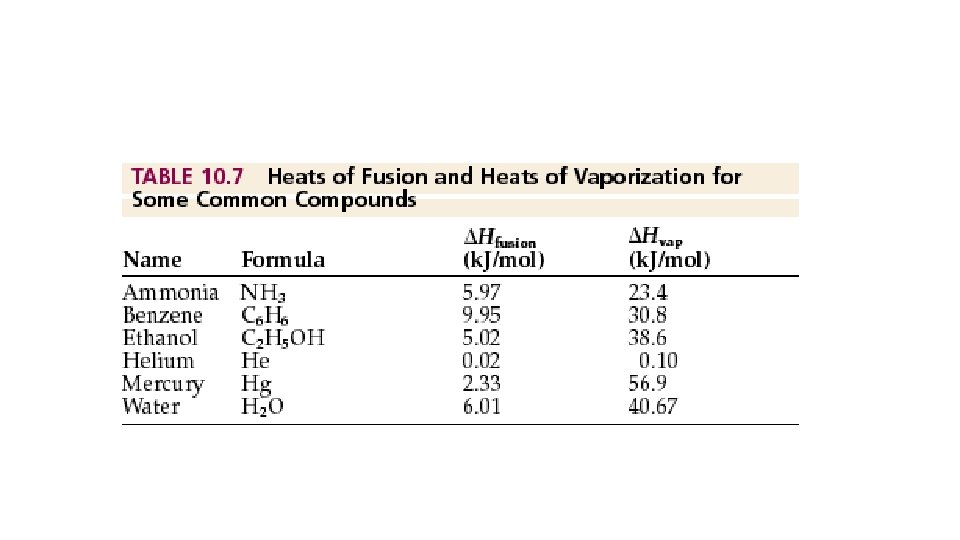
Diabatic process- heat(q) is exchanged between system and surr. (q = 0) • Diabatic processes can occur with or without a temperature change. • W/O temp. change: During a phase change: We will need: the heat of fusion(DHfus)- the amount of heat needed to melt or freeze a subst. Or The heat of vaporization(DHvap) – the amount of heat needed to boil or condense a subst. DHvap >> DHfus (always)

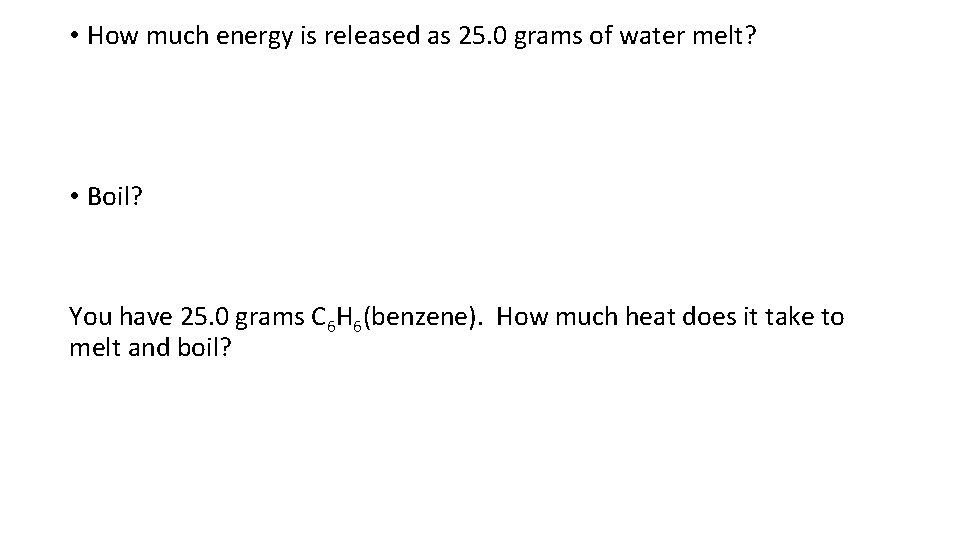
• How much energy is released as 25. 0 grams of water melt? • Boil? You have 25. 0 grams C 6 H 6(benzene). How much heat does it take to melt and boil?
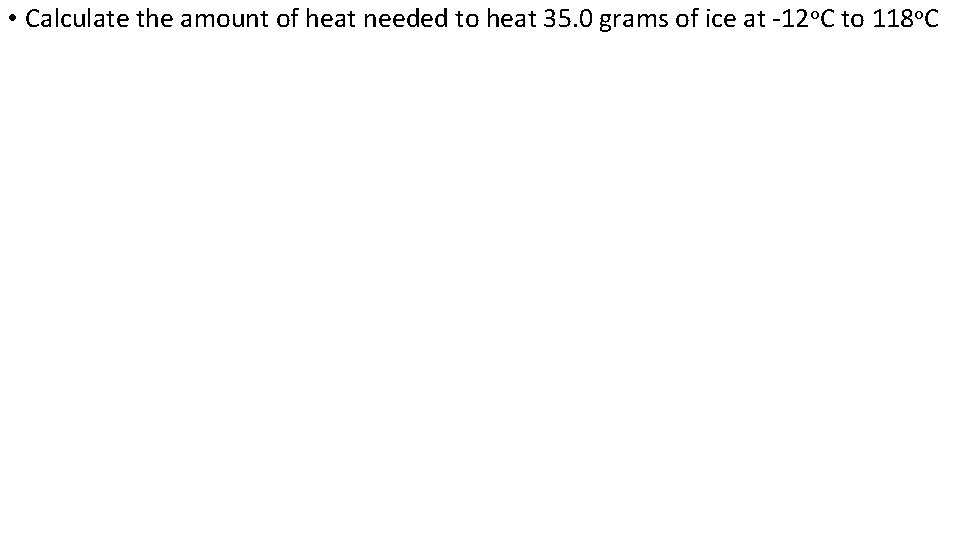
• Calculate the amount of heat needed to heat 35. 0 grams of ice at -12 o. C to 118 o. C

Hot & cold water mixed • 100. 0 grams of water at 25 o. C are mixed with 42. 7 grams of water at 37 o. C. What is the final temp. ? • 125 grams of water at 25 o. C are mixed with 62. 5 grams of water at 37 o. C. What is the final temp. ?

• 98. 5 grams of Al at 87 o. C are dropped into a calorimeter w/ 150. ml of water at 25 o. C. What is the equilibrium temp. of the system?

Hess’s Law-the DH of a process is always the same regardless of the number of steps taken. (state function) • Solve problems from handout

Standard Heat(enthalpy) of formation, DHf o The amount of heat gained/lost when forming 1 mole of compound from each free element, in it’s most stable state(phase and allotrope) • Write the reaction for the formation of each compound: • Na. Cl • H 2 SO 4 • C 2 H 6 • P 2 O 5

Standard Enthalpies of formation • Solve Problems from handout.



 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chemistry unit 7
Ap chemistry unit 7 General chemistry thermochemistry
General chemistry thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of
Thermochemistry is study of Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of
Thermochemistry is the study of Jelaskan pengertian cabang ilmu kimia termokimia
Jelaskan pengertian cabang ilmu kimia termokimia Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ