Thermochemistry Thermochemistry Study of energy changes that occur






- Slides: 16

Thermochemistry

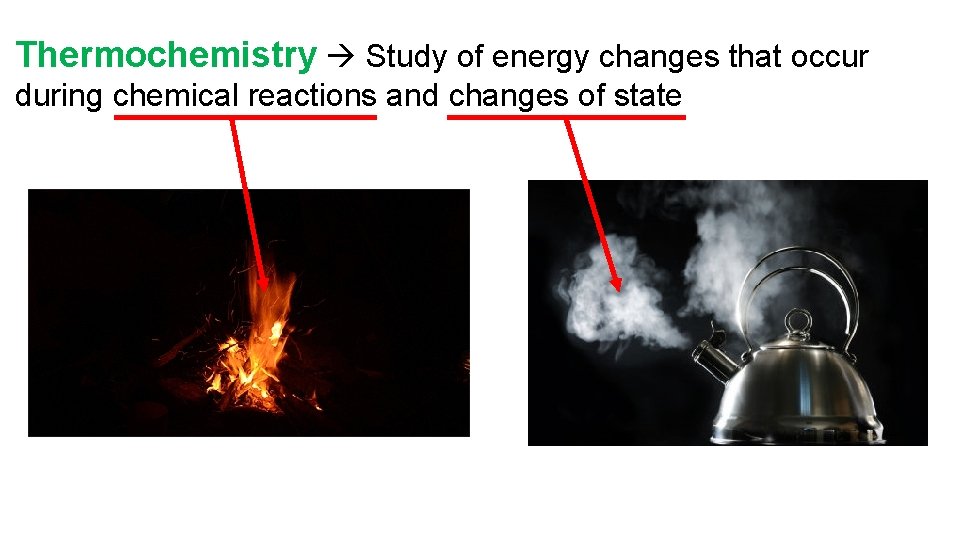
Thermochemistry Study of energy changes that occur during chemical reactions and changes of state

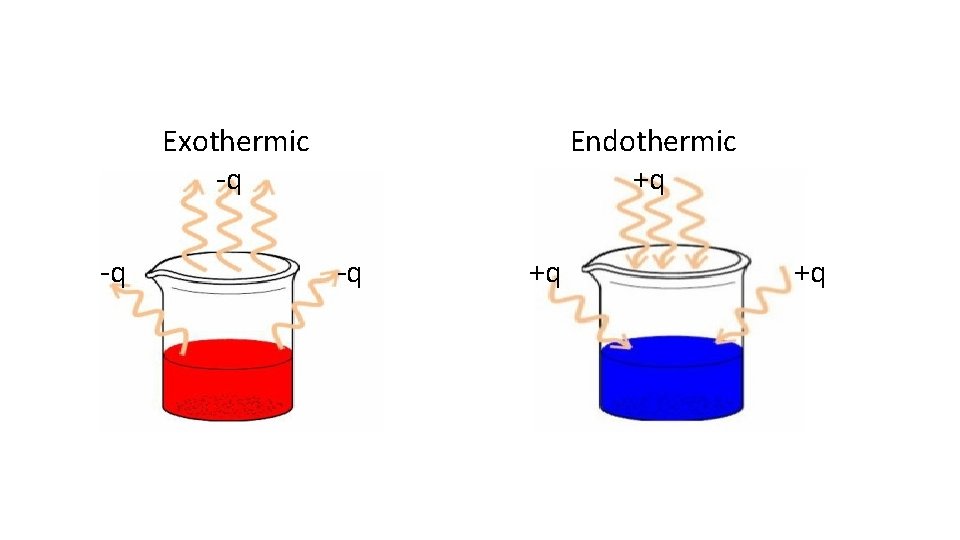
Endothermic Process that absorbs/gains heat Heat flows into the system (+q) Heat removed from the surroundings (-q) Surroundings feel cold

Exothermic Process that releases/produces heat Heat flows out of the system (-q) Heat added to the surroundings (+q) Surroundings may feel warm/hot

Exothermic -q -q Endothermic +q -q +q +q

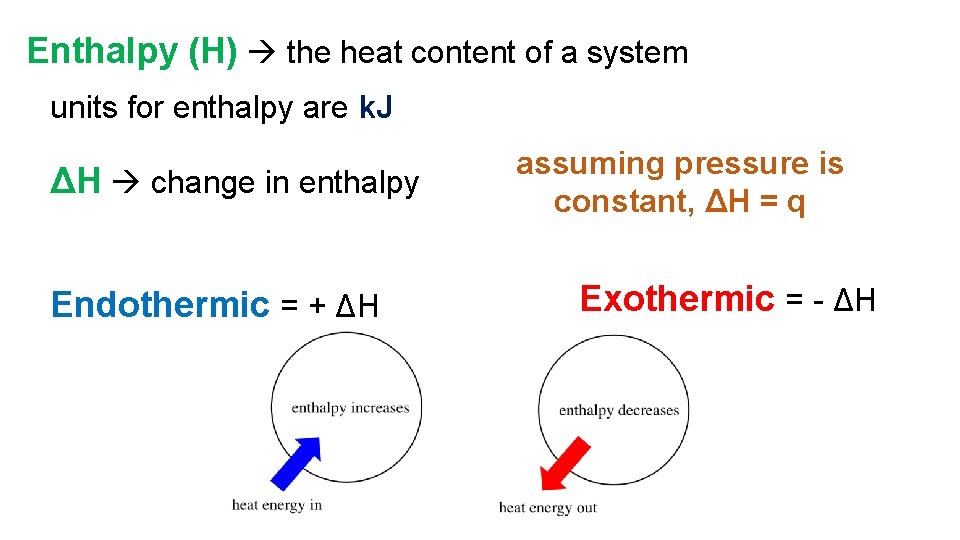
Enthalpy (H) the heat content of a system units for enthalpy are k. J ΔH change in enthalpy Endothermic = + ΔH assuming pressure is constant, ΔH = q Exothermic = - ΔH

ΔH & Chemical Reactions Chemical reactions always involves a change in heat energy

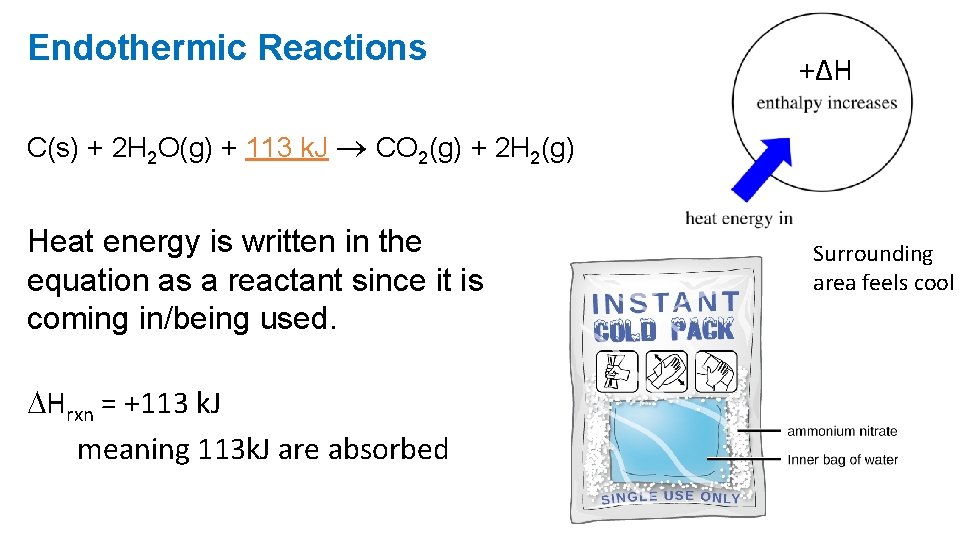
Endothermic Reactions +ΔH C(s) + 2 H 2 O(g) + 113 k. J CO 2(g) + 2 H 2(g) Heat energy is written in the equation as a reactant since it is coming in/being used. Hrxn = +113 k. J meaning 113 k. J are absorbed Surrounding area feels cool

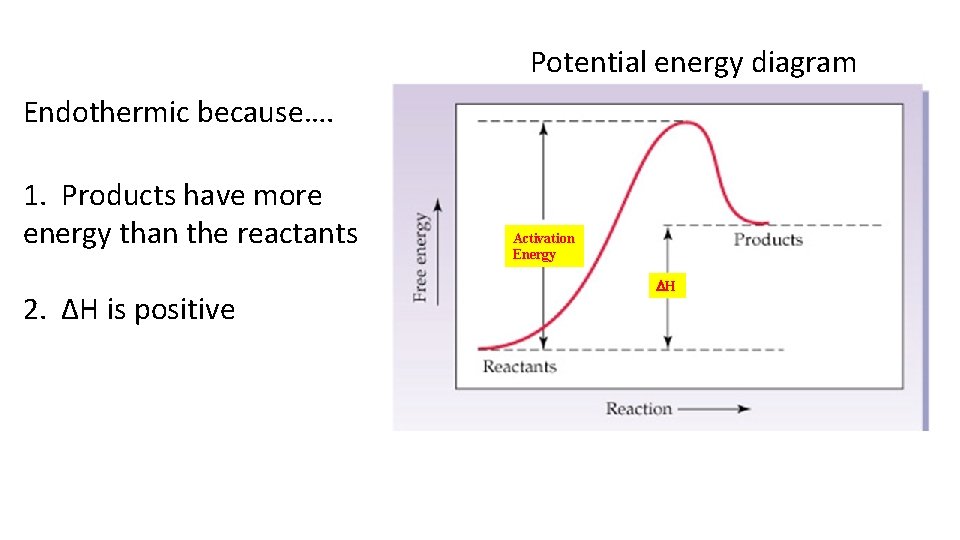
Potential energy diagram Endothermic because…. 1. Products have more energy than the reactants 2. ΔH is positive Activation Energy DH

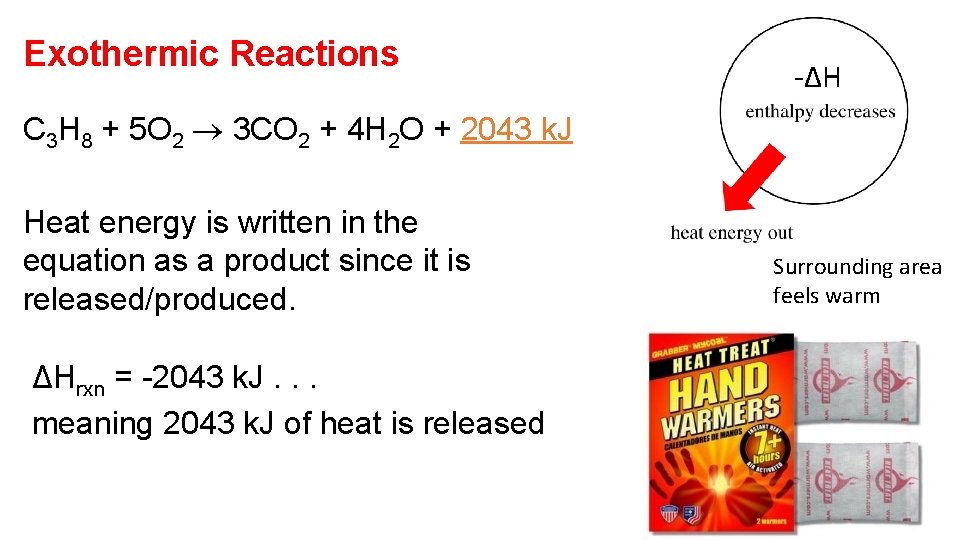
Exothermic Reactions -ΔH C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + 2043 k. J Heat energy is written in the equation as a product since it is released/produced. ΔHrxn = -2043 k. J. . . meaning 2043 k. J of heat is released Surrounding area feels warm

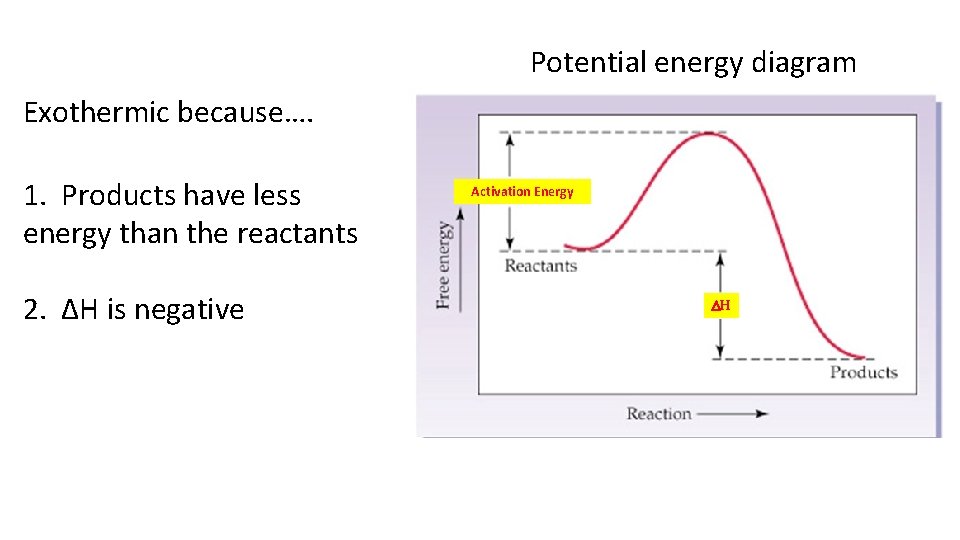
Potential energy diagram Exothermic because…. 1. Products have less energy than the reactants 2. ΔH is negative Activation Energy DH

Thermochemistry & Stoichiometry If you know the ΔH for a balanced equation, you may determine the amount of energy used or released by a reaction.

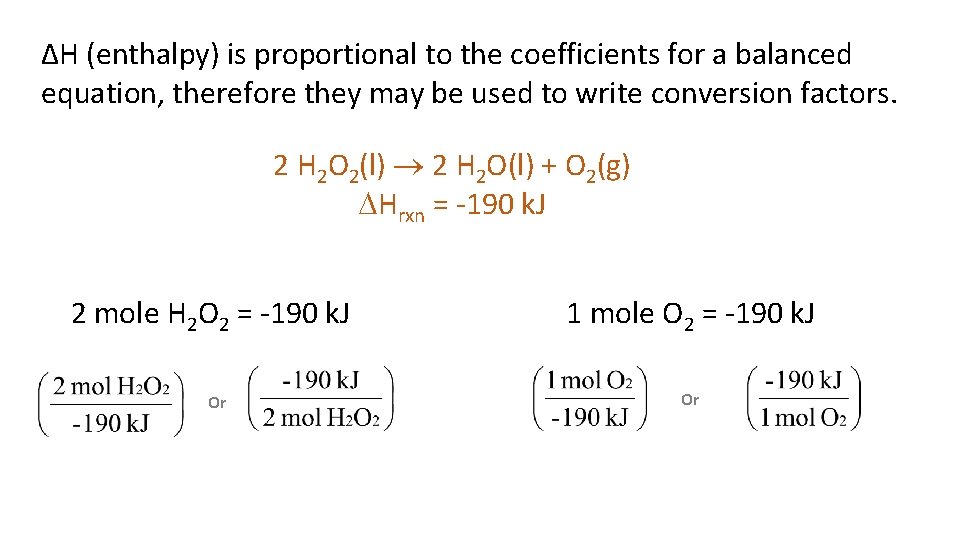
ΔH (enthalpy) is proportional to the coefficients for a balanced equation, therefore they may be used to write conversion factors. 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) Hrxn = -190 k. J 2 mole H 2 O 2 = -190 k. J Or 1 mole O 2 = -190 k. J Or

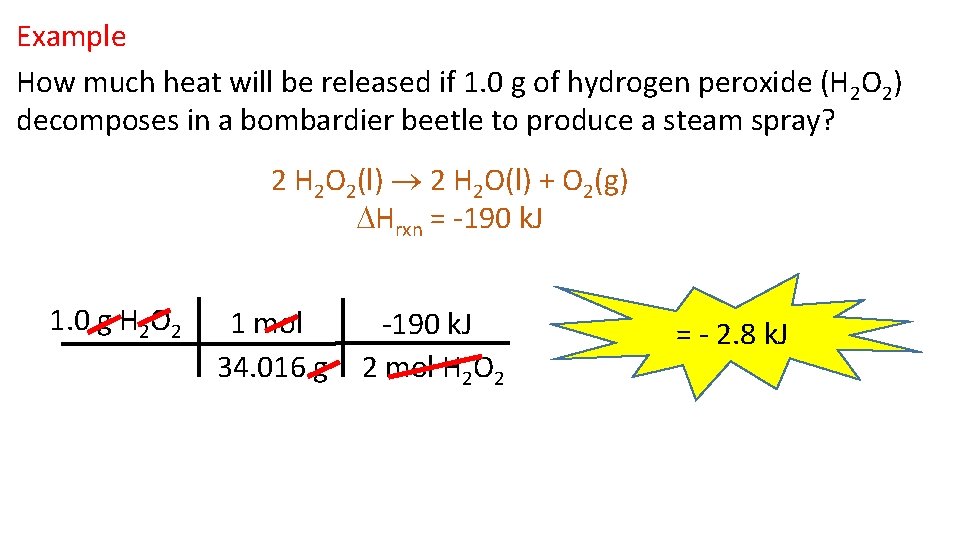
Example How much heat will be released if 1. 0 g of hydrogen peroxide (H 2 O 2) decomposes in a bombardier beetle to produce a steam spray? 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) Hrxn = -190 k. J 1. 0 g H 2 O 2 1 mol 34. 016 g -190 k. J 2 mol H 2 O 2 = - 2. 8 k. J

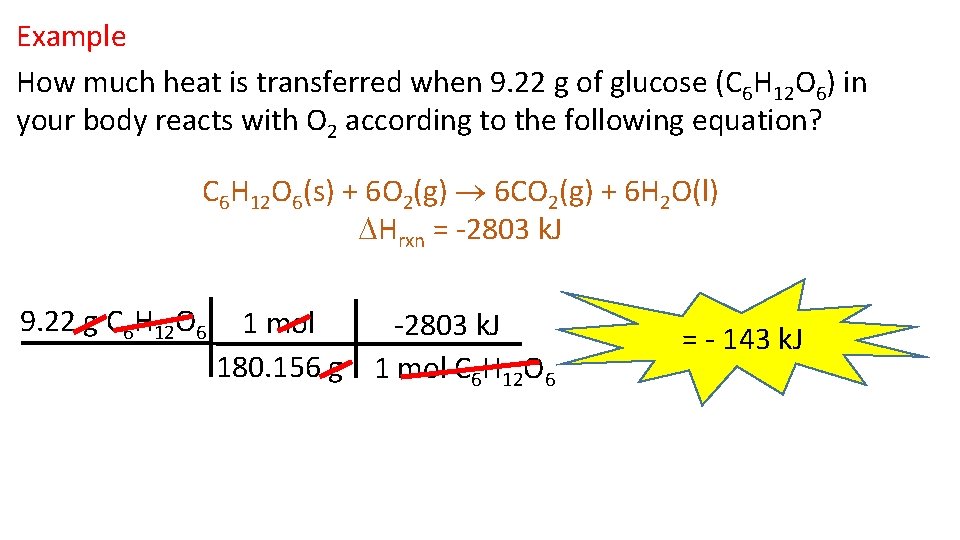
Example How much heat is transferred when 9. 22 g of glucose (C 6 H 12 O 6) in your body reacts with O 2 according to the following equation? C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) Hrxn = -2803 k. J 9. 22 g C 6 H 12 O 6 1 mol -2803 k. J 180. 156 g 1 mol C 6 H 12 O 6 = - 143 k. J

Example How much energy will be required to extract 59. 5 grams of tin? Sn. O 2(s) + 4 NO 2(g) + 2 H 2 O(l) + 192 k. J Sn(s) + 4 HNO 3(aq) Hrxn = +192 k. J 59. 5 g Sn 1 mol 118. 71 g 192 k. J 1 mol Sn = + 96. 2 k. J
 Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block nhĩ thất độ 3
Block nhĩ thất độ 3 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Changes in latitudes, changes in attitudes meaning
Changes in latitudes, changes in attitudes meaning Physicl change
Physicl change Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of