Thermochemistry Thermochemistry Study of energy changes that occur







- Slides: 29

Thermochemistry

Thermochemistry Study of energy changes that occur during chemical reactions and changes of state

Endothermic Process that absorbs/gains heat Heat flows into the system (+q) Heat removed from the surroundings (-q) Surroundings feel cold

Exothermic Process that releases/produces heat Heat flows out of the system (-q) Heat added to the surroundings (+q) Surroundings may feel warm/hot

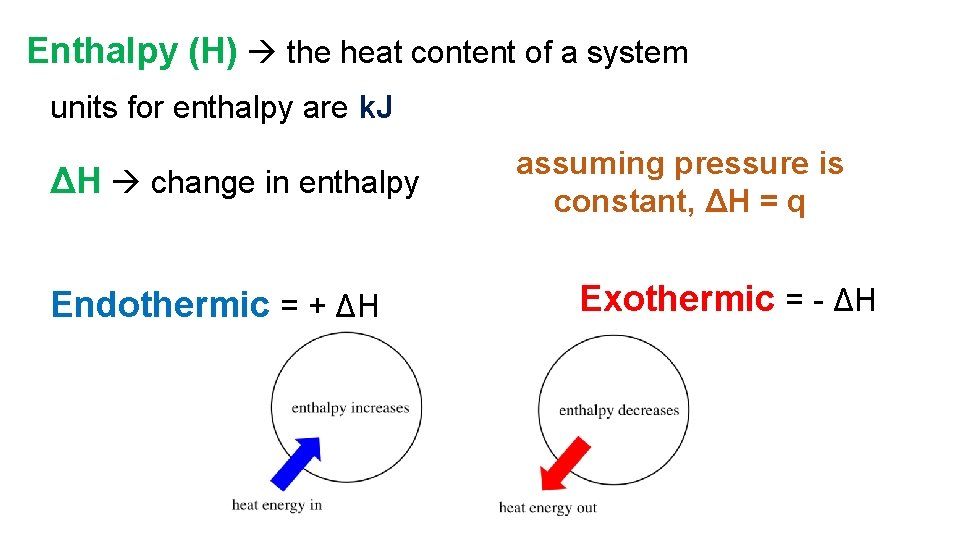
Enthalpy (H) the heat content of a system units for enthalpy are k. J ΔH change in enthalpy Endothermic = + ΔH assuming pressure is constant, ΔH = q Exothermic = - ΔH

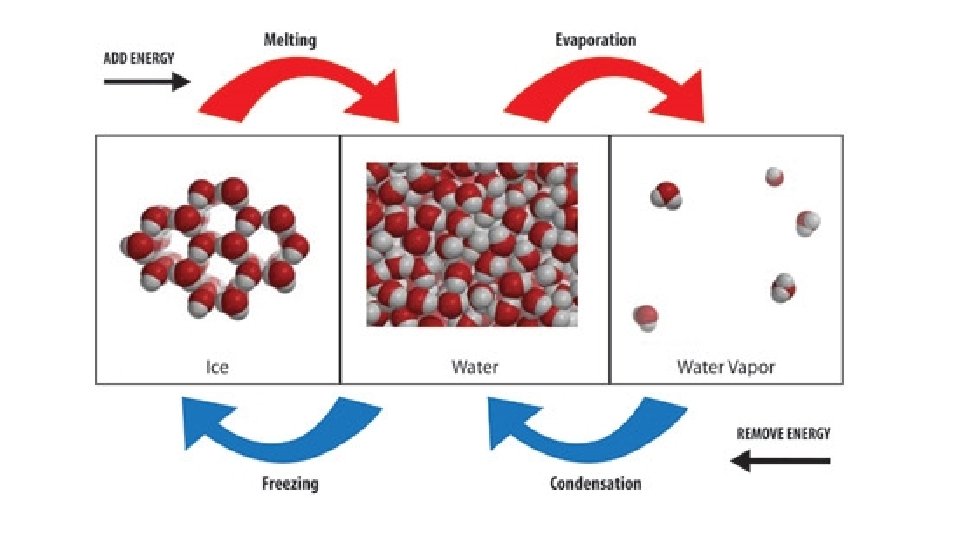
ΔH & Changes of State Change in state always involves a change in heat energy

Endothermic changes of state absorb heat energy, increasing in temperature Melting (fusion) Vaporization Sublimation

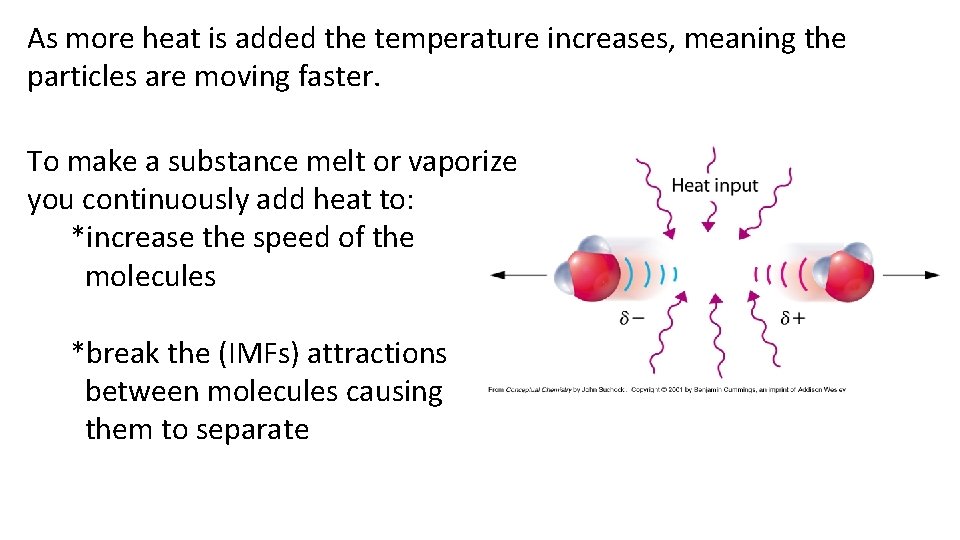
As more heat is added the temperature increases, meaning the particles are moving faster. To make a substance melt or vaporize you continuously add heat to: *increase the speed of the molecules *break the (IMFs) attractions between molecules causing them to separate

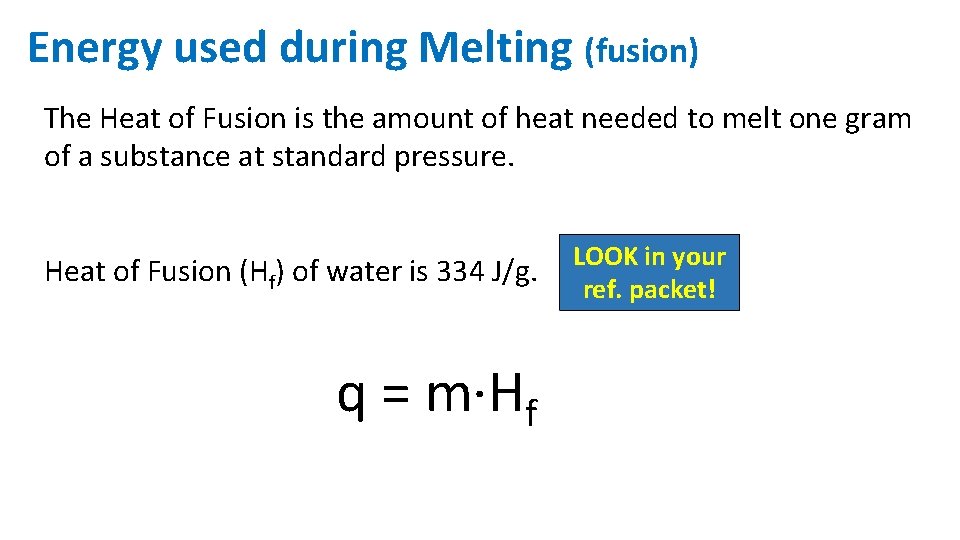
Energy used during Melting (fusion) The Heat of Fusion is the amount of heat needed to melt one gram of a substance at standard pressure. Heat of Fusion (Hf) of water is 334 J/g. q = m·Hf LOOK in your ref. packet!

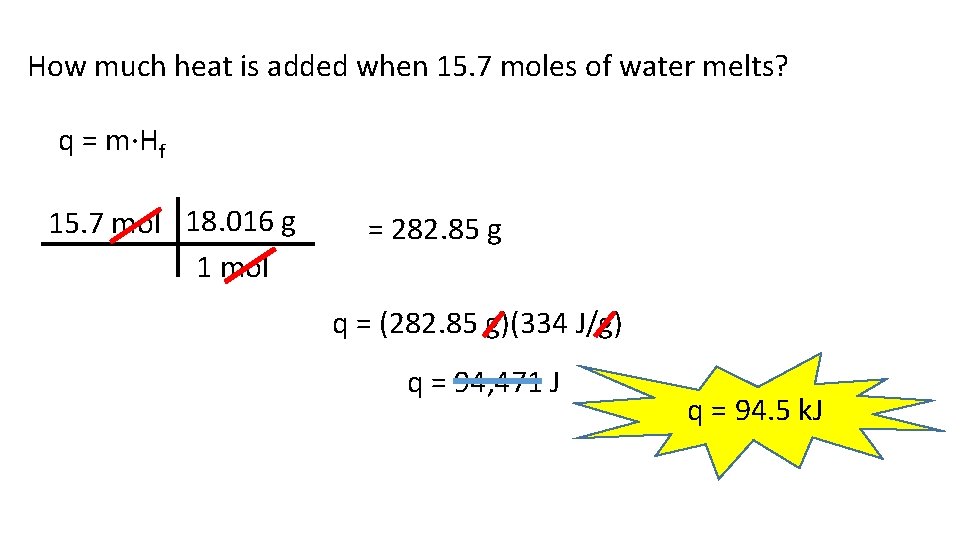
How much heat is added when 15. 7 moles of water melts? q = m·Hf 15. 7 mol 18. 016 g 1 mol = 282. 85 g q = (282. 85 g)(334 J/g) q = 94, 471 J q = 94. 5 k. J

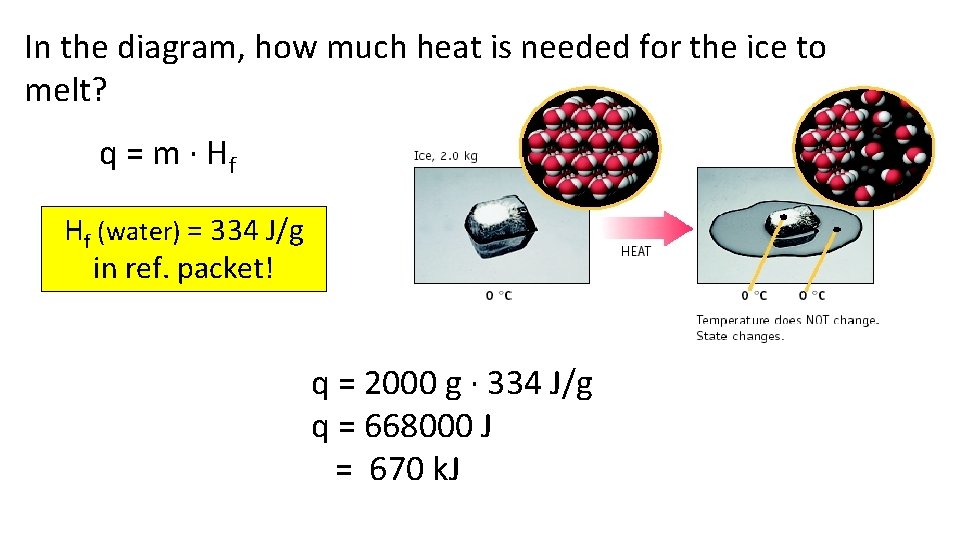
In the diagram, how much heat is needed for the ice to melt? q = m · Hf Hf (water) = 334 J/g in ref. packet! q = 2000 g · 334 J/g q = 668000 J = 670 k. J

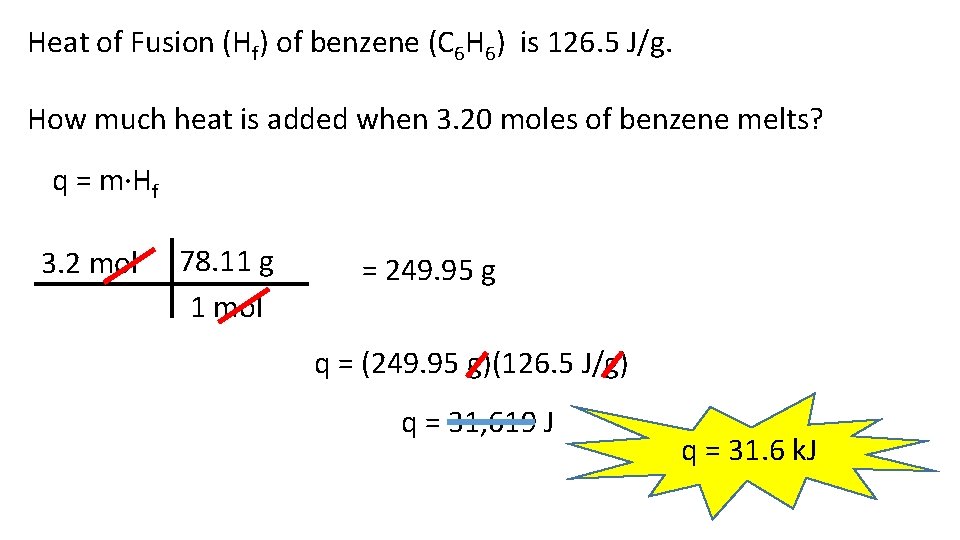
Heat of Fusion (Hf) of benzene (C 6 H 6) is 126. 5 J/g. How much heat is added when 3. 20 moles of benzene melts? q = m·Hf 3. 2 mol 78. 11 g 1 mol = 249. 95 g q = (249. 95 g)(126. 5 J/g) q = 31, 619 J q = 31. 6 k. J

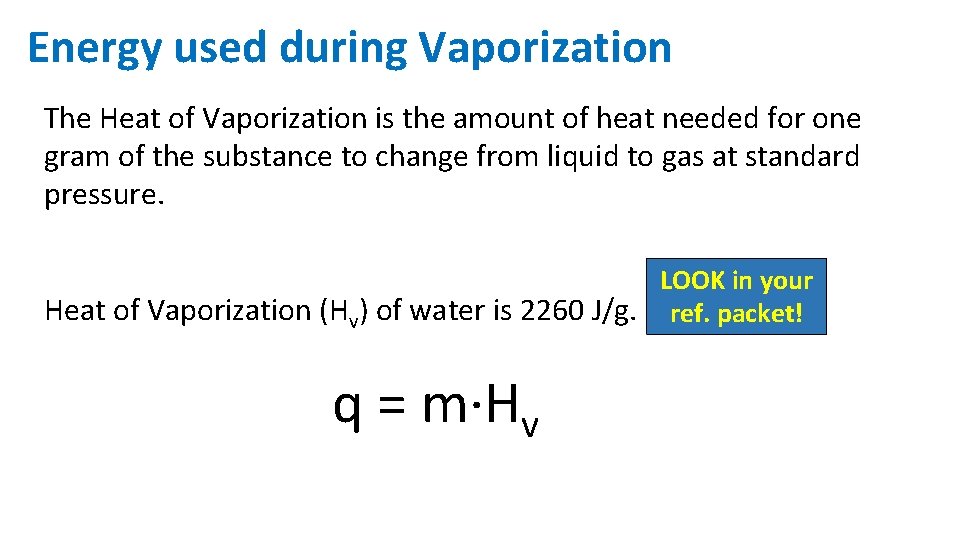
Energy used during Vaporization The Heat of Vaporization is the amount of heat needed for one gram of the substance to change from liquid to gas at standard pressure. LOOK in your Heat of Vaporization (Hv) of water is 2260 J/g. ref. packet! q = m·Hv

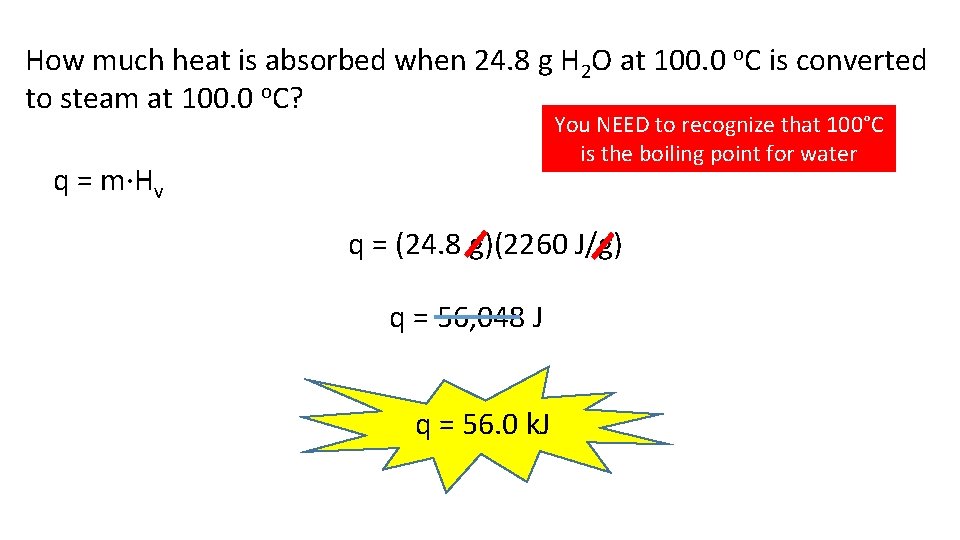
How much heat is absorbed when 24. 8 g H 2 O at 100. 0 o. C is converted to steam at 100. 0 o. C? You NEED to recognize that 100°C is the boiling point for water q = m·Hv q = (24. 8 g)(2260 J/g) q = 56, 048 J q = 56. 0 k. J

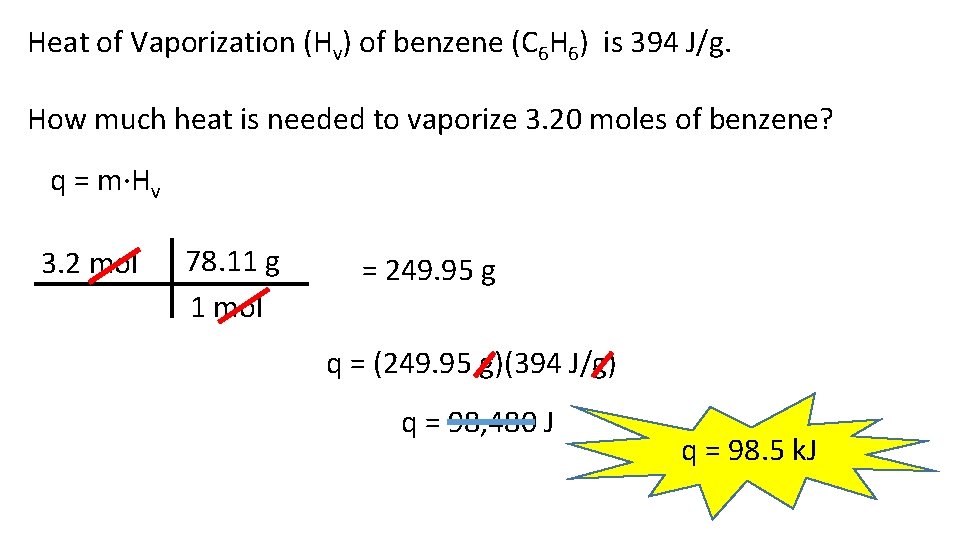
Heat of Vaporization (Hv) of benzene (C 6 H 6) is 394 J/g. How much heat is needed to vaporize 3. 20 moles of benzene? q = m·Hv 3. 2 mol 78. 11 g 1 mol = 249. 95 g q = (249. 95 g)(394 J/g) q = 98, 480 J q = 98. 5 k. J

Exothermic changes of state release heat energy, decreasing in temperature Freezing Condensation Deposition

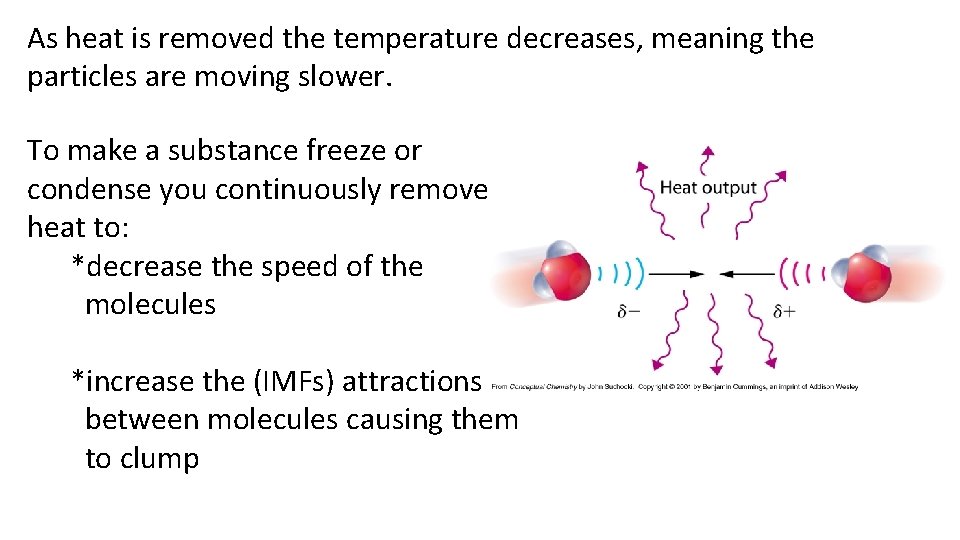
As heat is removed the temperature decreases, meaning the particles are moving slower. To make a substance freeze or condense you continuously remove heat to: *decrease the speed of the molecules *increase the (IMFs) attractions between molecules causing them to clump

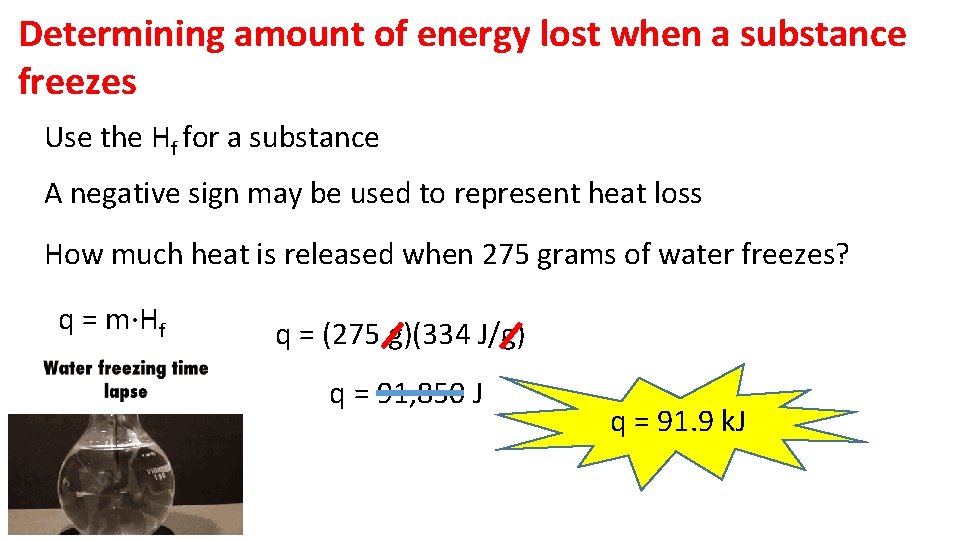
Determining amount of energy lost when a substance freezes Use the Hf for a substance A negative sign may be used to represent heat loss How much heat is released when 275 grams of water freezes? q = m·Hf q = (275 g)(334 J/g) q = 91, 850 J q = 91. 9 k. J

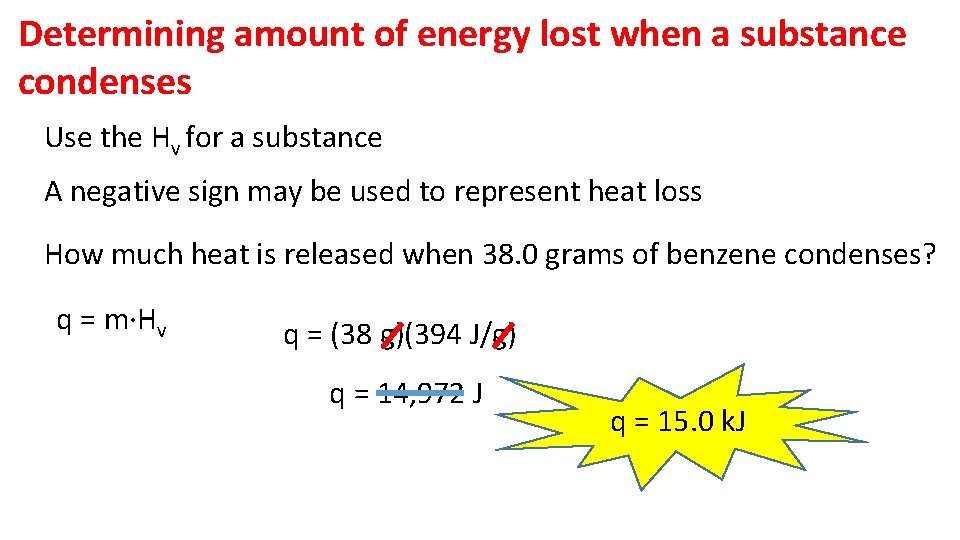
Determining amount of energy lost when a substance condenses Use the Hv for a substance A negative sign may be used to represent heat loss How much heat is released when 38. 0 grams of benzene condenses? q = m·Hv q = (38 g)(394 J/g) q = 14, 972 J q = 15. 0 k. J


ΔH & Heating/Cooling Curves

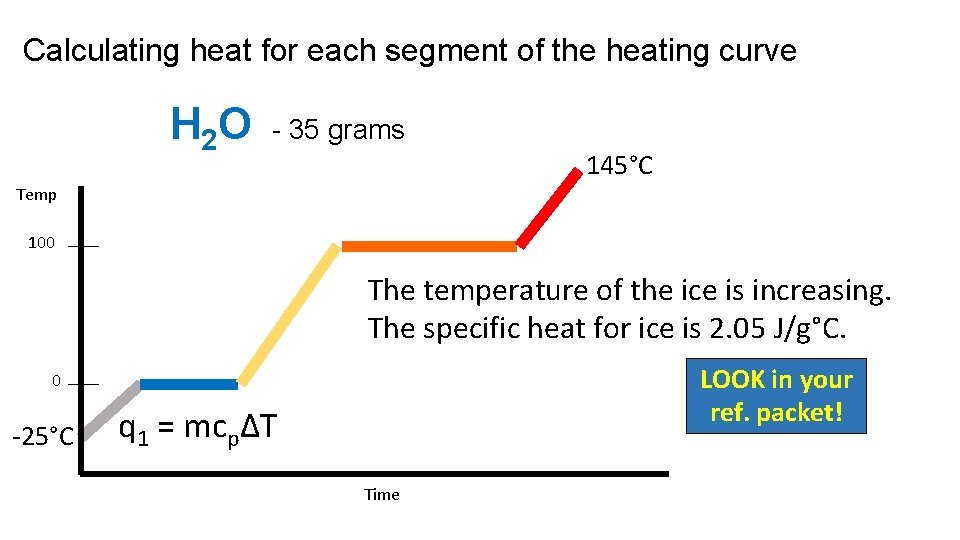
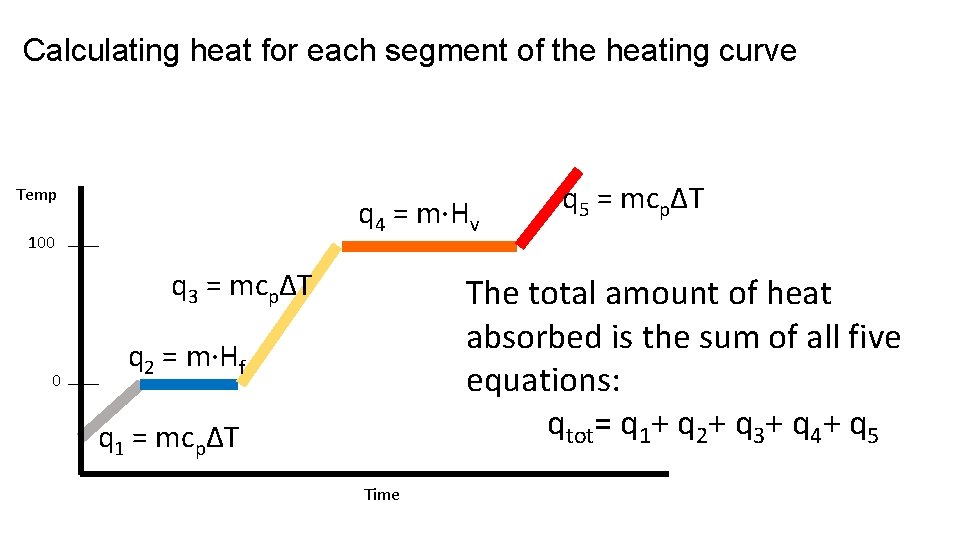
Calculating heat for each segment of the heating curve H 2 O - 35 grams 145°C Temp 100 The temperature of the ice is increasing. The specific heat for ice is 2. 05 J/g°C. LOOK in your ref. packet! 0 -25°C q 1 = mcpΔT Time

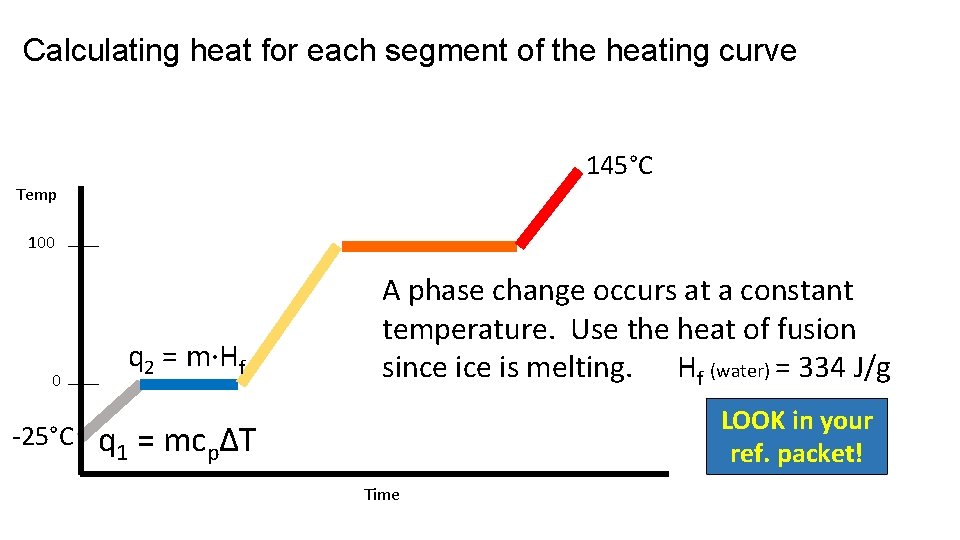
Calculating heat for each segment of the heating curve 145°C Temp 100 0 -25°C q 2 = m·Hf A phase change occurs at a constant temperature. Use the heat of fusion since is melting. Hf (water) = 334 J/g LOOK in your ref. packet! q 1 = mcpΔT Time

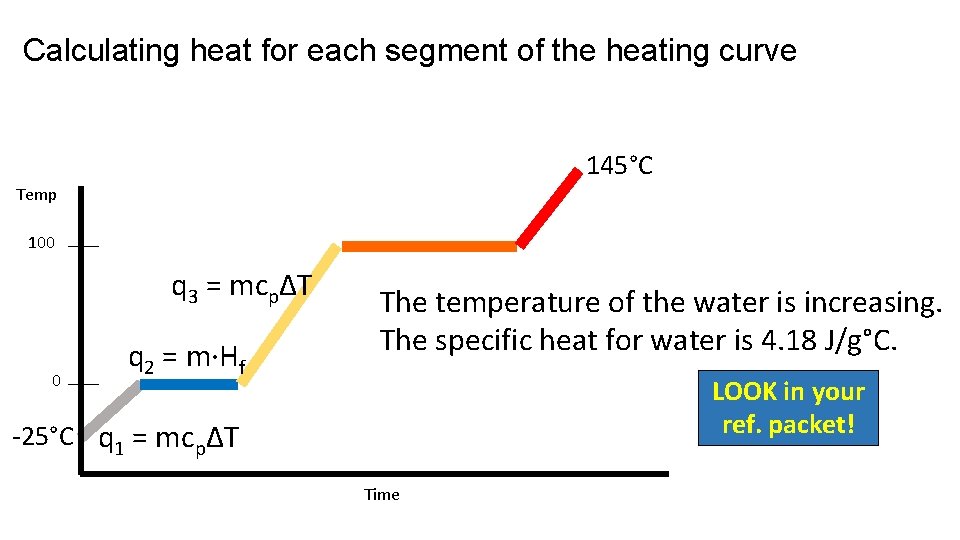
Calculating heat for each segment of the heating curve 145°C Temp 100 q 3 = mcpΔT 0 q 2 = m·Hf The temperature of the water is increasing. The specific heat for water is 4. 18 J/g°C. LOOK in your ref. packet! -25°C q 1 = mcpΔT Time

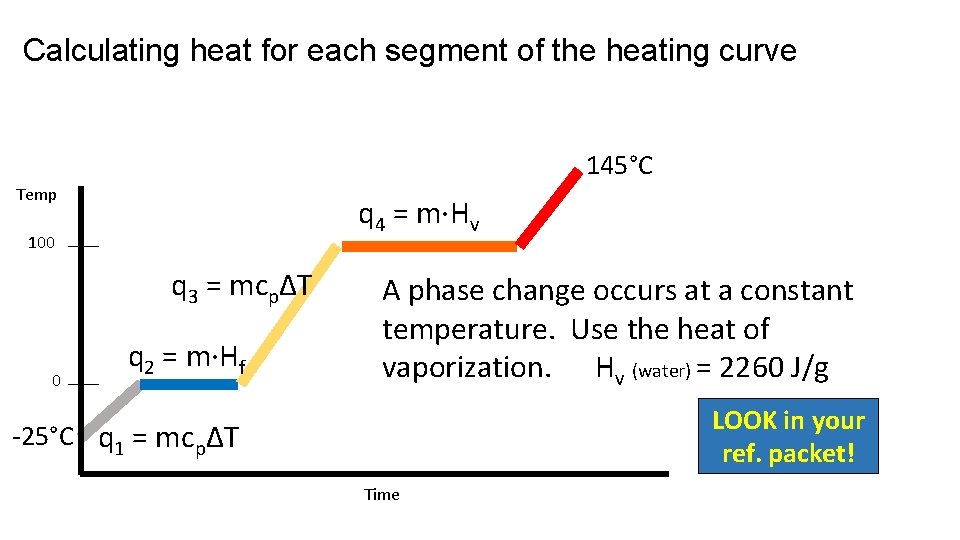
Calculating heat for each segment of the heating curve 145°C Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf A phase change occurs at a constant temperature. Use the heat of vaporization. Hv (water) = 2260 J/g LOOK in your ref. packet! -25°C q 1 = mcpΔT Time

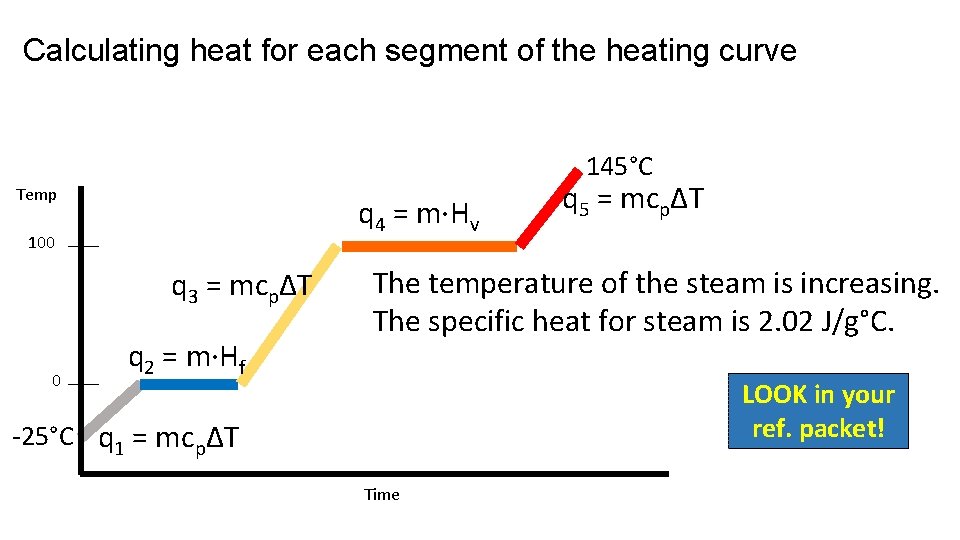
Calculating heat for each segment of the heating curve 145°C Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf q 5 = mcpΔT The temperature of the steam is increasing. The specific heat for steam is 2. 02 J/g°C. LOOK in your ref. packet! -25°C q 1 = mcpΔT Time

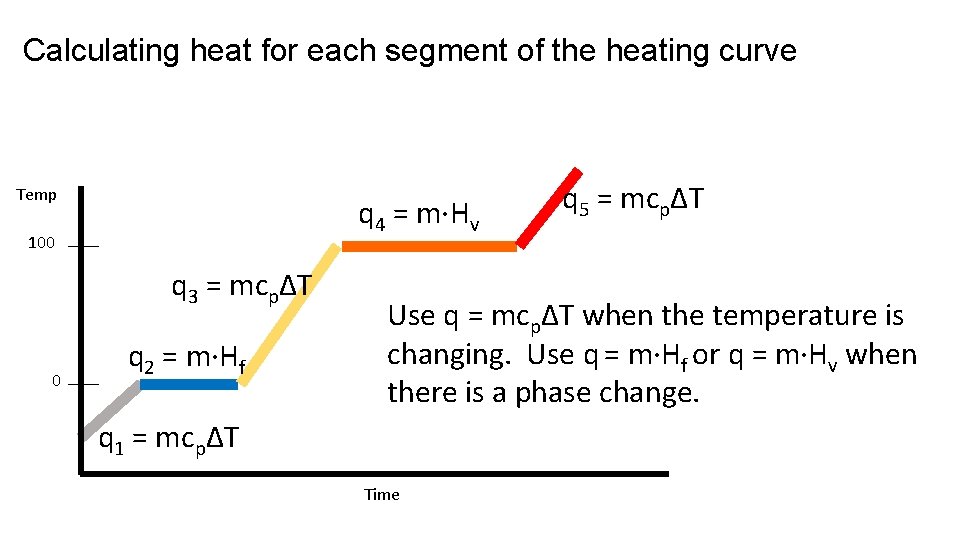
Calculating heat for each segment of the heating curve Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf q 5 = mcpΔT Use q = mcpΔT when the temperature is changing. Use q = m·Hf or q = m·Hv when there is a phase change. q 1 = mcpΔT Time

Calculating heat for each segment of the heating curve Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 5 = mcpΔT The total amount of heat absorbed is the sum of all five equations: qtot= q 1+ q 2+ q 3+ q 4+ q 5 q 2 = m·Hf q 1 = mcpΔT Time

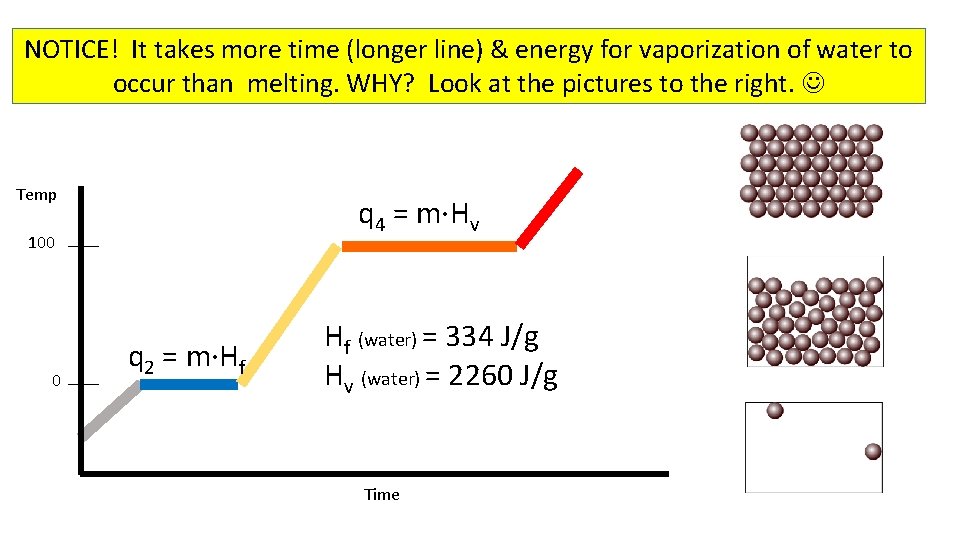
NOTICE! It takes more time (longer line) & energy for vaporization of water to occur than melting. WHY? Look at the pictures to the right. Temp q 4 = m·Hv 100 0 q 2 = m·Hf Hf (water) = 334 J/g Hv (water) = 2260 J/g Time