1 Thermochemistry Thermochemistry the study of the energy




- Slides: 16

1 Thermochemistry ¢ Thermochemistry the study of the energy changes that accompany physical or chemical changes in matter. ¢ Changes may be classified as physical, chemical, or nuclear ¢ Examples: l l l Ice melting Iron rusting An isotope used in medical therapy undergoes radioactive decay

2 Heat energy and Changes ¢ When discussing the transfer of heat energy, it is important to distinguish between the substances undergoing a change (chemical systems) and the system’s environment (surroundings) ¢ Chemical System: l ¢ A set of reactants and products under study, usually represented by a chemical equation Surroundings: l All matter around the system that is capable of absorbing or releasing thermal energy.

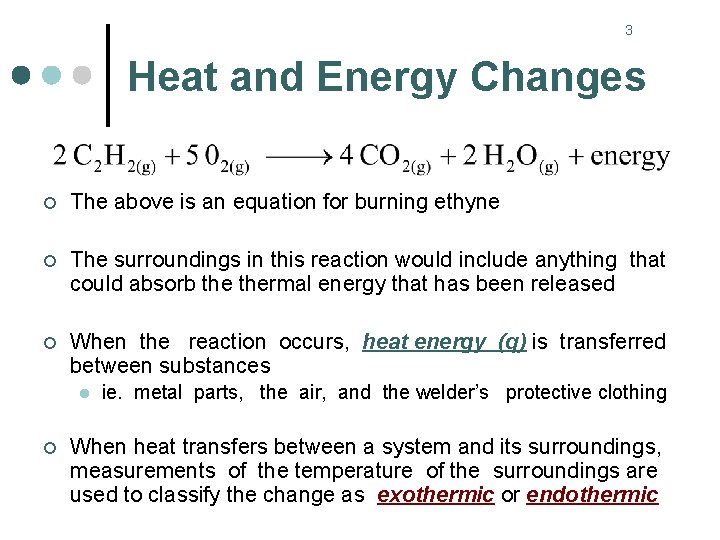
3 Heat and Energy Changes ¢ The above is an equation for burning ethyne ¢ The surroundings in this reaction would include anything that could absorb thermal energy that has been released ¢ When the reaction occurs, heat energy (q) is transferred between substances l ¢ ie. metal parts, the air, and the welder’s protective clothing When heat transfers between a system and its surroundings, measurements of the temperature of the surroundings are used to classify the change as exothermic or endothermic

4 Heat &Temperature ¢ Heat energy (q): l l l ¢ measure of the total kinetic energy in an object (and so depends on how large the object is, or how much of it there is) The amount of energy transferred between substances it is represented by the symbol q with units of Joules (J) Temperature: l l Measure of the Average kinetic energy of the particles in a sample of matter measured most often in (o. C, K)

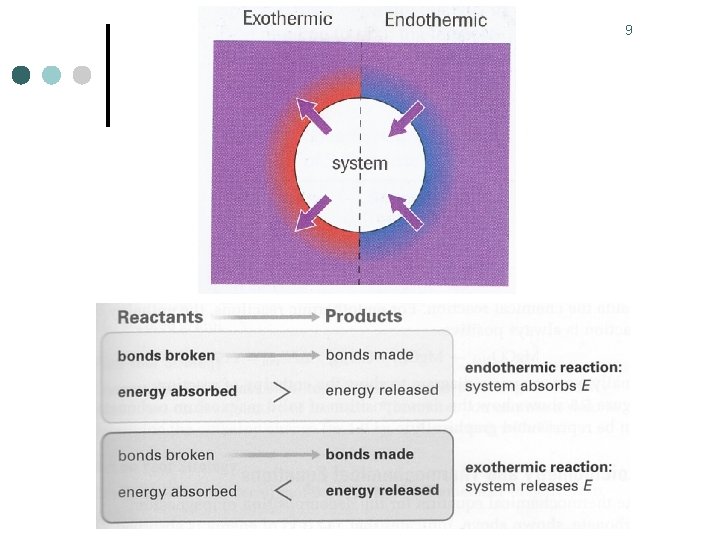
5 Exothermic & Endothermic Reactions ¢ Exothermic reaction l l ¢ Releasing thermal energy as heat flows out of the system Kinetic energy of the surrounding molecules increases temperature increases Endothermic reaction l l Absorbing thermal energy as heat flows into the system Kinetic energy of the surrounding molecules decreases temperature decreases

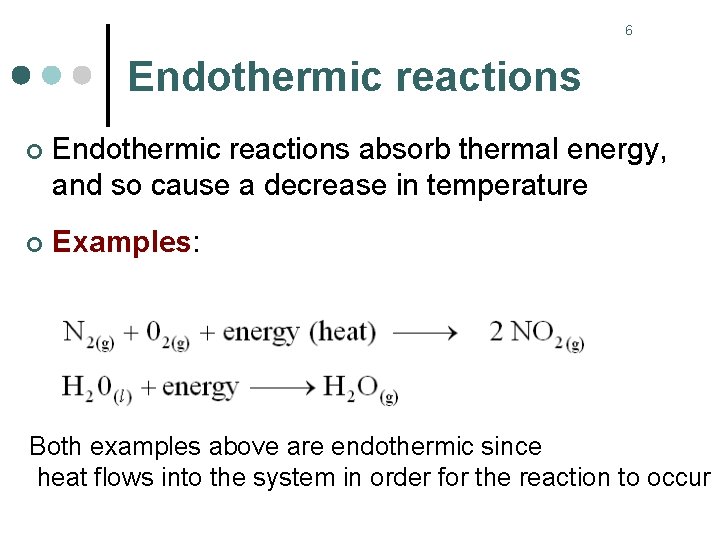
6 Endothermic reactions ¢ Endothermic reactions absorb thermal energy, and so cause a decrease in temperature ¢ Examples: Both examples above are endothermic since heat flows into the system in order for the reaction to occur

7 Exothermic reactions release heat energy to their surroundings ¢ They cause the temperature of the surrounding to increase ¢ Examples ¢ Combustion ¢ Neutralization ¢ Reactions of metals with water ¢

8 Exothermic Reactions ¢ This combustion reaction is exothermic because heat flows into the surroundings ¢ Notice the heat energy is being produced by the reaction, & transferred to the surroundings ¢ Once heat is transferred to the surroundings, it is used to increase thermal energy of the molecules in the surroundings ¢ Molecules in the surroundings have greater kinetic energy, the temperature of the surroundings will increase.

9

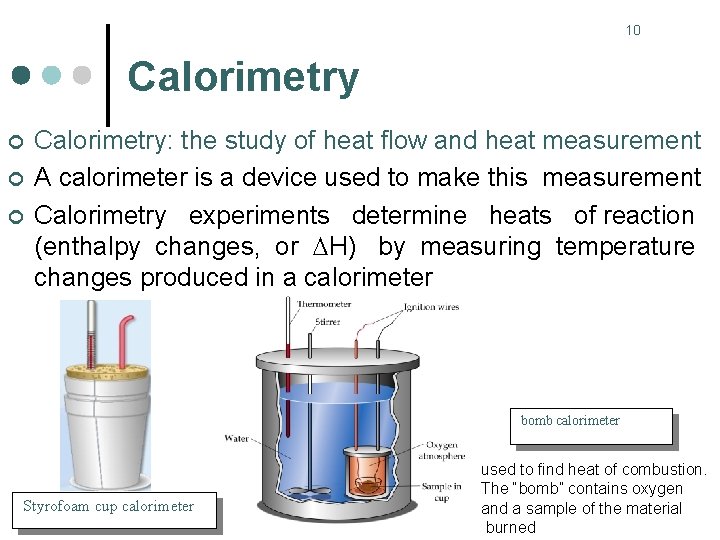
10 Calorimetry ¢ ¢ ¢ Calorimetry: the study of heat flow and heat measurement A calorimeter is a device used to make this measurement Calorimetry experiments determine heats of reaction (enthalpy changes, or H) by measuring temperature changes produced in a calorimeter bomb calorimeter Styrofoam cup calorimeter used to find heat of combustion. The “bomb” contains oxygen and a sample of the material burned

Measuring Energy Changes ¢ The amount of heat energy given by a substance will depend on: l l l ¢ 11 Mass (in grams) Temperature Change ( T) Type of Substance These factors can be combined into an equation to represent the quantity of heat (q) transferred…

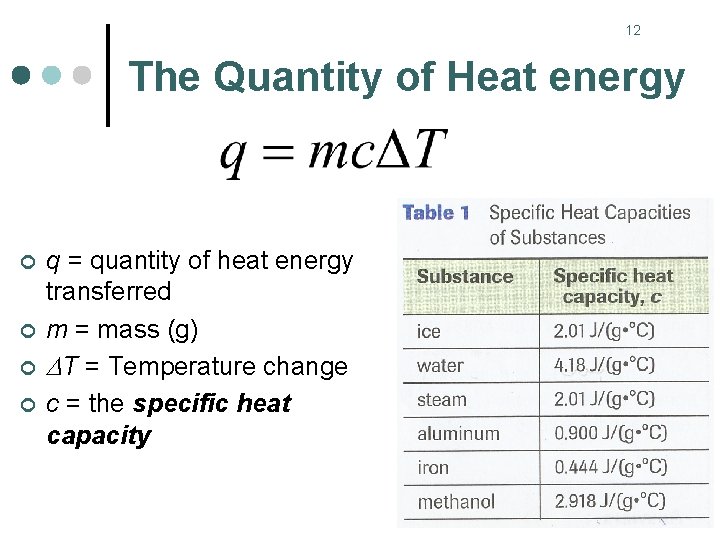
12 The Quantity of Heat energy ¢ ¢ q = quantity of heat energy transferred m = mass (g) T = Temperature change c = the specific heat capacity

13 How did we arrive at q = mc T? Important Properties: Heat Capacity and Specific Heat Capacity ¢ The thermal properties of a substance are those that describe its ability to absorb or release heat without changing chemically ¢ They include heat capacity and specific heat capacity.

14 Heat Capacity ¢ The heat capacity of an object is the amount of heat needed to raise its temperature by 1 C ¢ Heat Capacity = Heat absorbed T (1) ex: The heat capacity of a cup of water at 18 C is the # of joules of energy needed to raise its temperature to 19 C

15 Ex. 1: Calculating Quantity of Heat ¢ What would the final temperature be if 250. 0 J of heat were transferred into 10. 0 g of methanol initially at 20 0 C ?

16 Calculating Quantity of Heat ¢ ¢ ¢ Q 2 When 600 m. L of water in an electric kettle is heated from 20 0 C to 85 0 C to make a cup of tea, how much heat flows into the water? Q 3 Calculate the heat absorbed by 250 ml of water to raise its temperature from 20. 0 0 C to 50. 0 0 C. The specific heat capacity of water is 4. 18 J/(g· 0 C) Q 4 Iron metal has a specific heat capacity of 0. 449 J/(g· 0 C). How much heat is transferred to a 5. 0 g piece of iron, initially at 20. 0 0 C, when it is placed in a pot of boiling water? The final temperature of the metal is 100 0 C.
 Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is the study of
Thermochemistry is the study of Study of energy transformations
Study of energy transformations Termokimia adalah ilmu yang mempelajari tentang
Termokimia adalah ilmu yang mempelajari tentang Thermochemistry is the study of
Thermochemistry is the study of Q = m x cp x ∆t
Q = m x cp x ∆t Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Gibbs free energy unit
Gibbs free energy unit Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Balanced thermochemical equation
Balanced thermochemical equation Thermochemistry
Thermochemistry Thermochemistry gaussian
Thermochemistry gaussian