Thermochemistry Energy in Reactions Thermochemistry Most chemical reactions


- Slides: 7

Thermochemistry Energy in Reactions

Thermochemistry • Most chemical reactions involve a change in energy. Energy is either absorbed by the reaction or released by the reaction. • Endothermic Reaction – energy is absorbed in the reaction; heat is treated as a reactant • Exothermic Reaction – energy is released in the reaction; heat is treated as a product.

Thermochemistry • The energy in these reactions is measure in kilojoules (k. J). By placing the amount of heat lost or gained in the reaction we can treat heat as any other stoichiometric calculation.

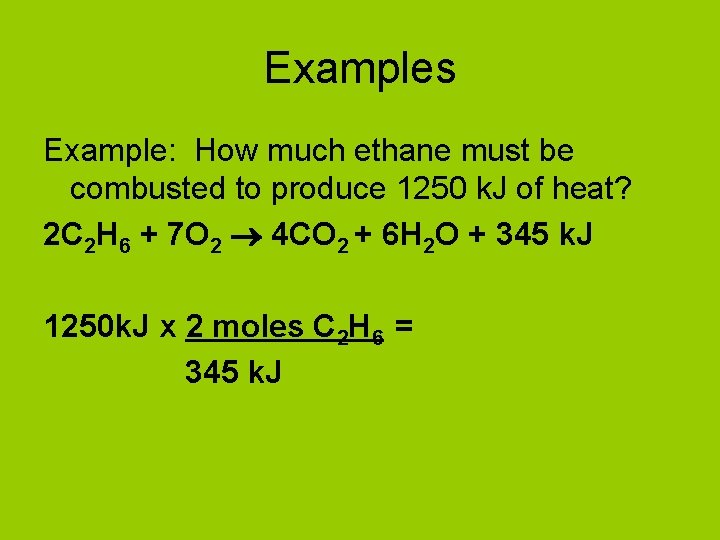
Examples Example: How much ethane must be combusted to produce 1250 k. J of heat? 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O + 345 k. J 1250 k. J x 2 moles C 2 H 6 = 345 k. J

Examples Example: How much ethane must be combusted to produce 1250 k. J of heat? 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O + 345 k. J 1250 k. J x 2 moles C 2 H 6 = 7. 25 moles C 2 H 6 345 k. J

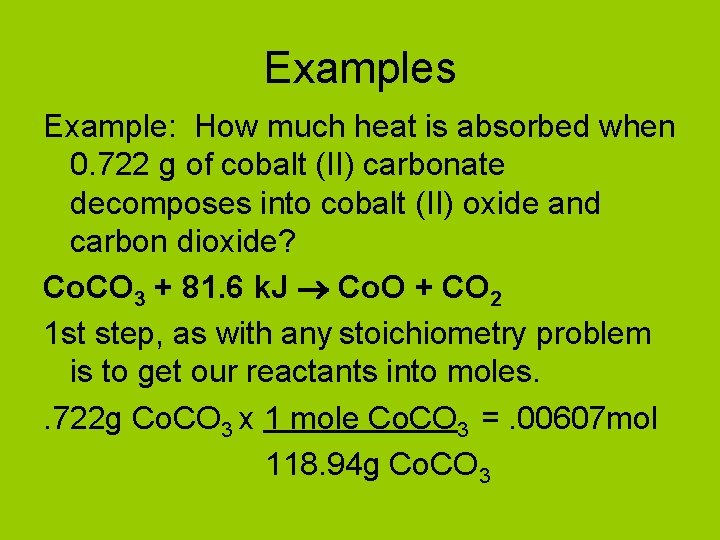
Examples Example: How much heat is absorbed when 0. 722 g of cobalt (II) carbonate decomposes into cobalt (II) oxide and carbon dioxide? Co. CO 3 + 81. 6 k. J Co. O + CO 2 1 st step, as with any stoichiometry problem is to get our reactants into moles. . 722 g Co. CO 3 x 1 mole Co. CO 3 =. 00607 mol 118. 94 g Co. CO 3

Example: How much heat is absorbed when 0. 722 g of cobalt (II) carbonate decomposes into cobalt (II) oxide and carbon dioxide? Co. CO 3 + 81. 6 k. J Co. O + CO 3 Now we solve like any other stoichiometry problem. . 00607 mol Co. CO 3 x 81. 6 k. J =. 495 k. J 1 mole Co. CO 3
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Apoenzyme
Apoenzyme Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry