Thermochemistry Chemical Energy Reactions Thermochemistry Study of heat
















- Slides: 24

Thermochemistry Chemical Energy & Reactions

Thermochemistry • Study of heat changes occuring during reactions – Potential • Stored energy due to chemical composition – Kinetic • is the energy of motion – Heat/thermal • Energy transferred from one substance to another due to temperature differences. • Always from hot ---> cold – Chemical • Energy stored within bonds of molecules (intramolecular) and between molecules (intermolecular) – Nuclear Energy • Change in the nucleus of the atom to form different elements

Energy and changes • System – Defined as the part of the universe on which we focus our attention. – The SURROUNDINGS include everything else in the universe.

Temperature vs Heat • Temperature – Measure of how hot or cold a substance is. – Higher T indicates higher molecular motion – Measured in o. C or K • Heat – Form of energy contained in a substance – Unit in J (Joule) or KJ (Kilojoule) – Compare T and heat content of a cup of coffee vs. iceberg – Quantity of heat in a substance depends on its: • Temperature • Mass • Specific Heat

• Enthalpy (H) – Energy content (KJ) of a substance – Excluding nuclear energy • Heat of Reaction ( H) – Amount of energy (KJ) released or absorbed during a chemical reaction or physical change • Heat of formation – Energy involved when a substance is produced • Heat of combustion – Energy released when a substance is burned

Types of changes • Molar heat of… – Refers to the amount of energy released or absorbed per mole of a substance involved in a chemical reaction. • Each type of change absorbs or releases energy. – Least in physical- most in nuclear (by far)

Exothermic reaction • A process that releases heat to the surroundings (becomes hot to touch) • Ex- combustion, condensation • Represented in equations by 2 H 2 + O 2 ---> 2 H 2 O + 340 k. J 2 H 2 + O 2 ---> 2 H 2 O H = -340 k. J • Negative sign denotes exothermic

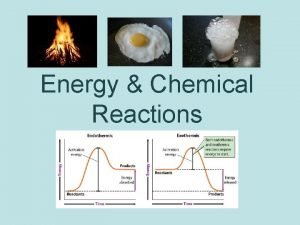
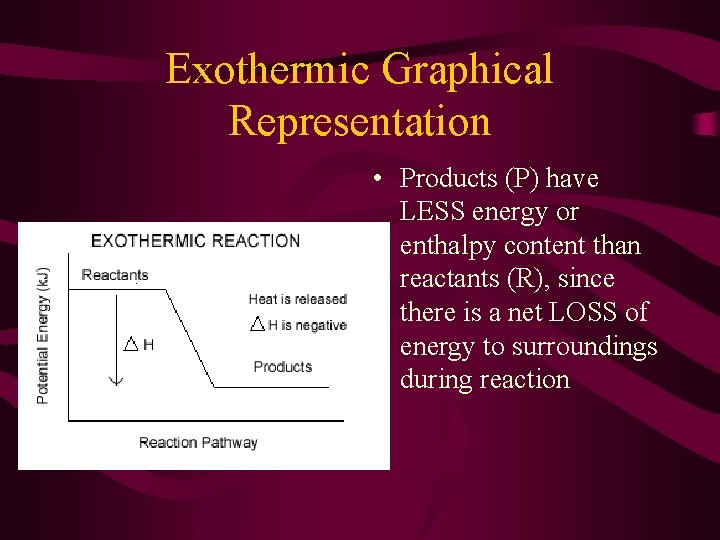
Exothermic Graphical Representation • Products (P) have LESS energy or enthalpy content than reactants (R), since there is a net LOSS of energy to surroundings during reaction

Endothermic Reactions • Involve a net absorption of energy by the reactants. • Ex- Recharging a battery, evaporation • Represented by: N 2 + 2 O 2 + 67. 6 k. J --> 2 NO 2 N 2 + 2 O 2 ---> 2 NO 2 H = +67. 6 k. J • Positive sign indicates an endothermic reaction

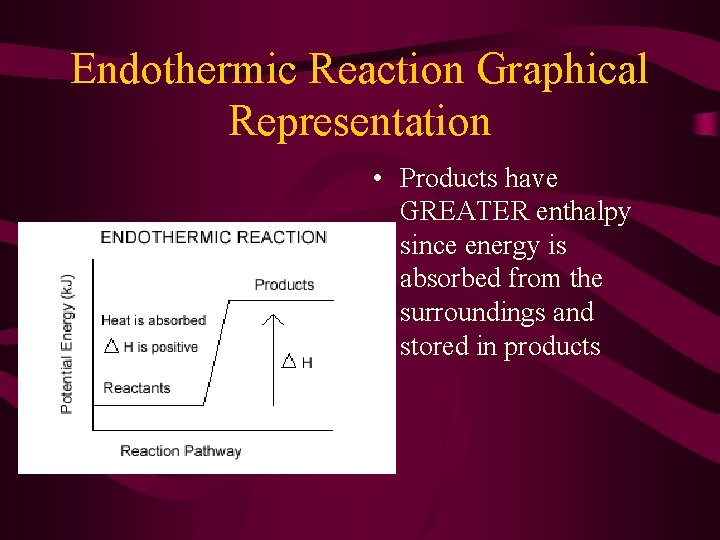
Endothermic Reaction Graphical Representation • Products have GREATER enthalpy since energy is absorbed from the surroundings and stored in products

Specific Heat Capacity (c) • Amount of heat energy required to raise or lower the temperature of 1 g of a substance by 10 C. • Ex– – – H 2 O= 4. 19 J/go. C Iron = 0. 45 J/go. C Aluminum = 0. 89 J/go. C Copper = 0. 387 J/go. C Brass = 0. 38 J/go. C Zinc = 0. 39 J/go. C

How to find heat absorbed or lost? • Use Q = mc T • Where – m = mass of substance – Q = heat energy (KJ) – T = Change in temperature (Tf - Ti) – C = specific heat of substance

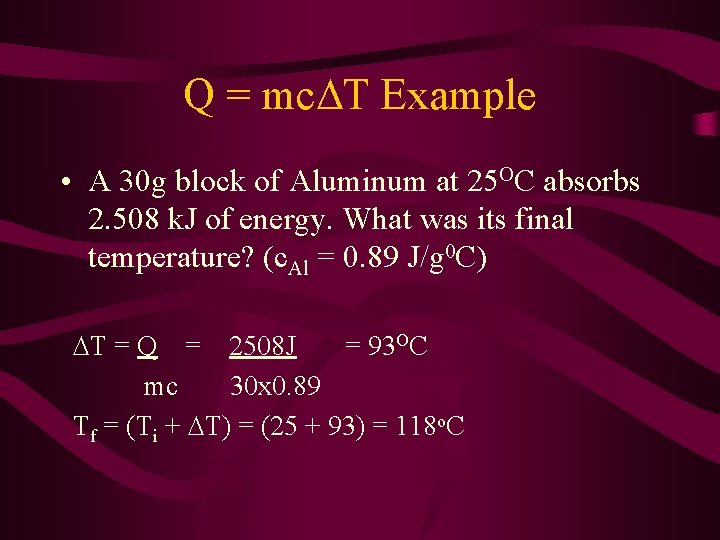
Q = mc T Example • A 30 g block of Aluminum at 25 OC absorbs 2. 508 k. J of energy. What was its final temperature? (c. Al = 0. 89 J/g 0 C) T = Q = 2508 J = 93 OC mc 30 x 0. 89 Tf = (Ti + T) = (25 + 93) = 118 o. C

Calorimeter • An instrument used to measure the amount of heat energy released or absorbed during a chemical reaction • If the reaction is EXOthermic, heat will be released into the water and the temperature of the H 2 O (TH 2 O) will rise • If the reaction is ENDOthermic, heat will be absorbed from the H 2 O and TH 2 O drops.

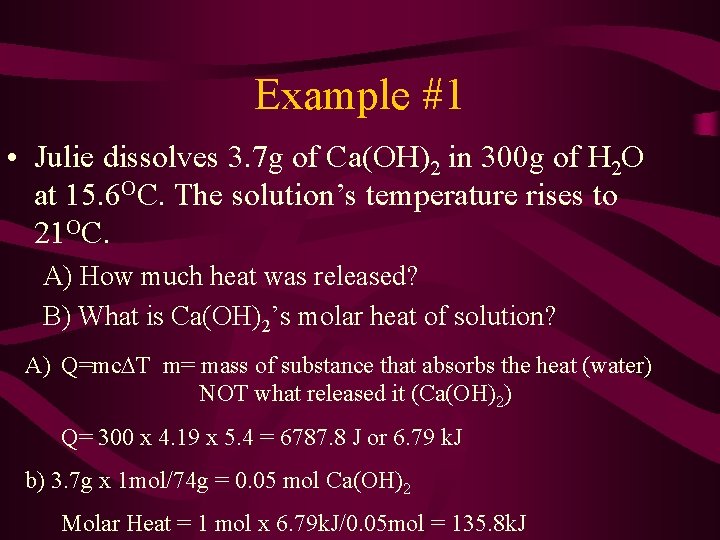
Example #1 • Julie dissolves 3. 7 g of Ca(OH)2 in 300 g of H 2 O at 15. 6 OC. The solution’s temperature rises to 21 OC. A) How much heat was released? B) What is Ca(OH)2’s molar heat of solution? A) Q=mc T m= mass of substance that absorbs the heat (water) NOT what released it (Ca(OH)2) Q= 300 x 4. 19 x 5. 4 = 6787. 8 J or 6. 79 k. J b) 3. 7 g x 1 mol/74 g = 0. 05 mol Ca(OH)2 Molar Heat = 1 mol x 6. 79 k. J/0. 05 mol = 135. 8 k. J

Example #2 • When 2. 4 g of CH 4 is burned in a calorimeter, the 850 m. L of H 2 O at 19 OC rises to 25. 3 OC. What is the molar heat of combustion of CH 4? Q = mc T = 850 x 4. 19 x 6. 3 = 22 437. 5 J or 22. 44 k. J/2. 4 g x 1 mol/16 g = 0. 15 mol CH 4 1 mol x 22. 44 k. J/0. 15 mol = 149. 6 k. J

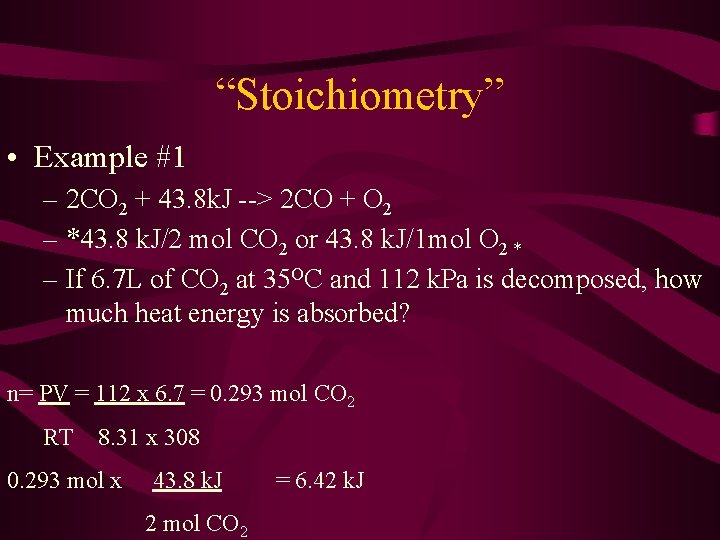
“Stoichiometry” • Example #1 – 2 CO 2 + 43. 8 k. J --> 2 CO + O 2 – *43. 8 k. J/2 mol CO 2 or 43. 8 k. J/1 mol O 2 * – If 6. 7 L of CO 2 at 35 OC and 112 k. Pa is decomposed, how much heat energy is absorbed? n= PV = 112 x 6. 7 = 0. 293 mol CO 2 RT 8. 31 x 308 0. 293 mol x 43. 8 k. J 2 mol CO 2 = 6. 42 k. J

Example #2 • Using N 2 + 3 H 2 ---> 2 NH 3 H= -34. 2 k. J • If 22. 23 k. J of heat is released in a reaction, how many grams of H 2 must be reacted?

22. 23 k. J x 3 H 2 = 1. 95 mol H 2 34. 2 k. J 1. 95 mol H 2 x 2 g/mol = 3. 9 g of H 2 must be reacted

Heat Transfer • Heat always transfers from hot to cold • Heat will continue to transfer from the hot object to the cold object (as long as they are in contact) until the same final temperature is reached. • Heat LOST by hot = heat GAINED by cold • -Qlost hot = +Qgained cold • -(mh x Th x ch) = +(mc x Tc x cc)

Example • A 450 g sample of water at 80 OC is mixed with 360 g of water at 12 o. C. What is the final temperature of the mixture? -[450 g x (Tf - 80 OC) x 4. 19] = + [360 g x (Tf - 12 OC) x 4. 19] 4. 19’s cancel eachother out -450 Tf + 36000 = 360 Tf - 4320 -810 Tf = -40320 Tf = 49. 78 OC

Heat of neutralization problem • When 50 m. L of a 0. 25 M Na. OH solution is neutralized by 100 m. L of an HCl solution, the temperature of the mixture rises to 1. 5 OC. What is the molar heat of neutralization of Na. OH? (assume densities and specific heat of the solutions are same as water)

Solution • Heat released into “mixture”: Q=mc T = 150 g x 1. 5 OC x 4. 19 J/g. OC = 942. 8 J Mols of Na. OH neutralized : (molarity =mol/Vol) mol = molarity x Volume 0. 25 mol/L x 0. 05 L = 0. 0125 mol Na. OH Molar heat of neutralization of Na. OH: • 1 mol Na. OH x 942. 8 J/0. 0125 mol = 75420 J/mol or 75. 42 k. J/mol

Homework Read pages 653 -671 (Pay attention to graph on page 668) Questions within pages #1 -8 (due next class) Questions page 681 #13 -29
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chemical reactions study guide
Chemical reactions study guide Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Apoenzyme is
Apoenzyme is 10 examples of redox reaction
10 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of
Thermochemistry is the study of Ilmu kimia yang mempelajari tentang kalor reaksi
Ilmu kimia yang mempelajari tentang kalor reaksi Thermochemistry is study of
Thermochemistry is study of Q = m x cp x delta t
Q = m x cp x delta t How are thermal energy and temperature different
How are thermal energy and temperature different Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Energy example
Energy example