Chemical Reactions A Chemical Reactions 1 Chemical reactions



- Slides: 24

Chemical Reactions

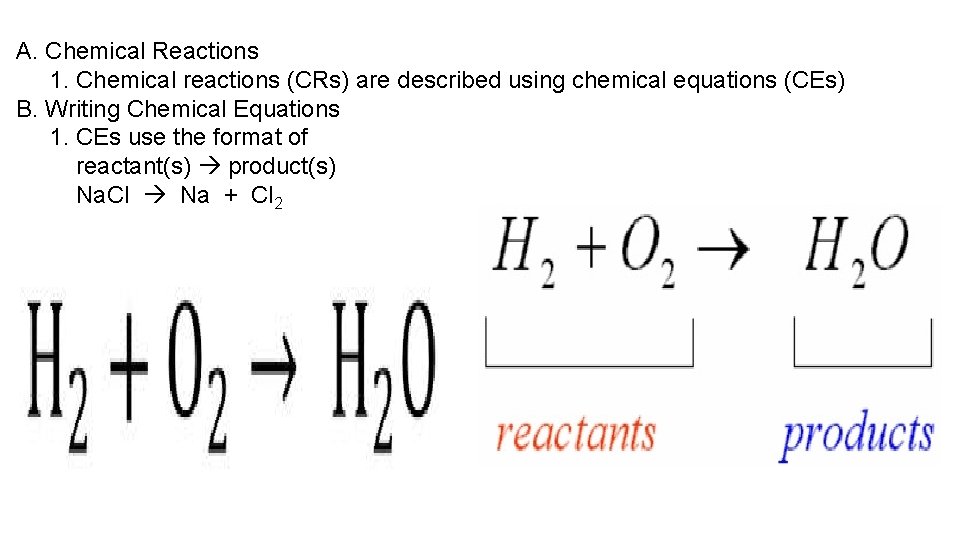
A. Chemical Reactions 1. Chemical reactions (CRs) are described using chemical equations (CEs) B. Writing Chemical Equations 1. CEs use the format of reactant(s) product(s) Na. Cl Na + Cl 2

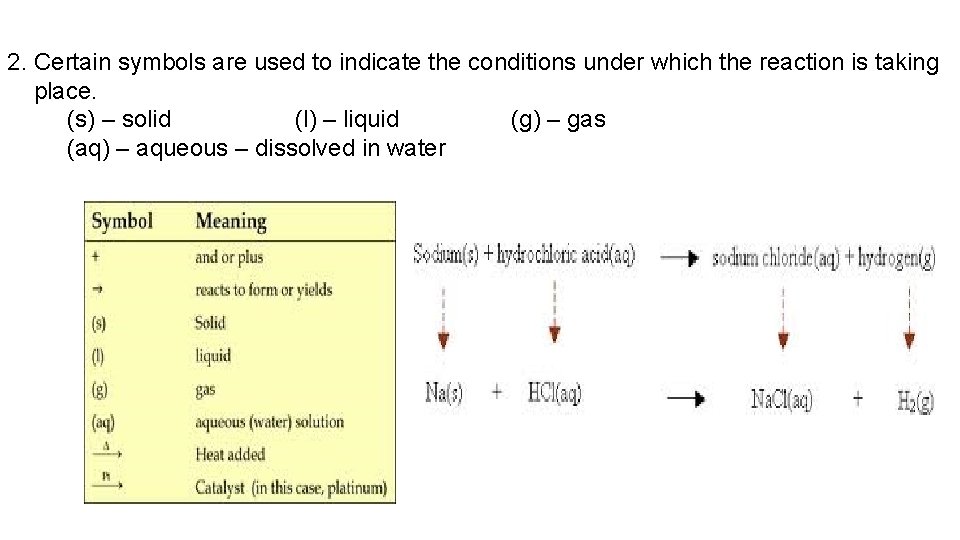
2. Certain symbols are used to indicate the conditions under which the reaction is taking place. (s) – solid (l) – liquid (g) – gas (aq) – aqueous – dissolved in water

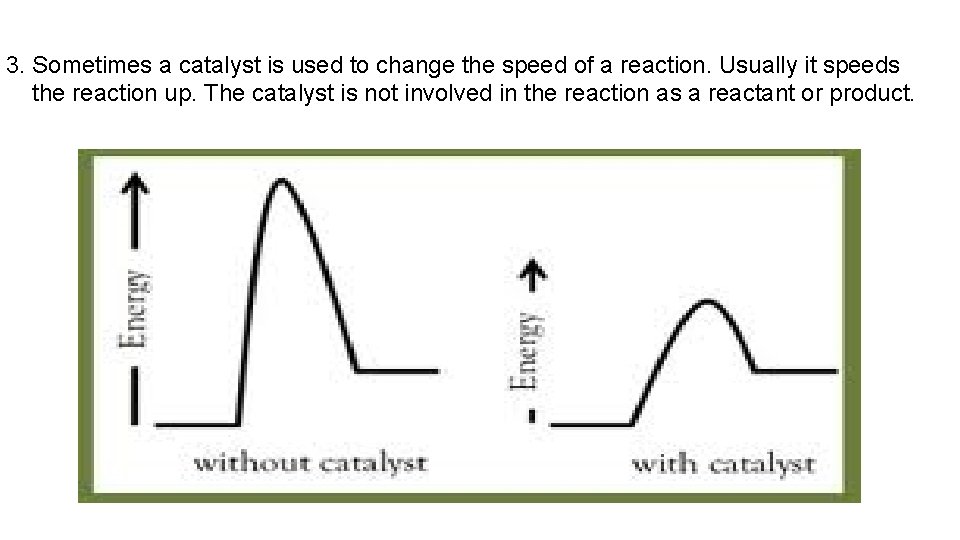
3. Sometimes a catalyst is used to change the speed of a reaction. Usually it speeds the reaction up. The catalyst is not involved in the reaction as a reactant or product.

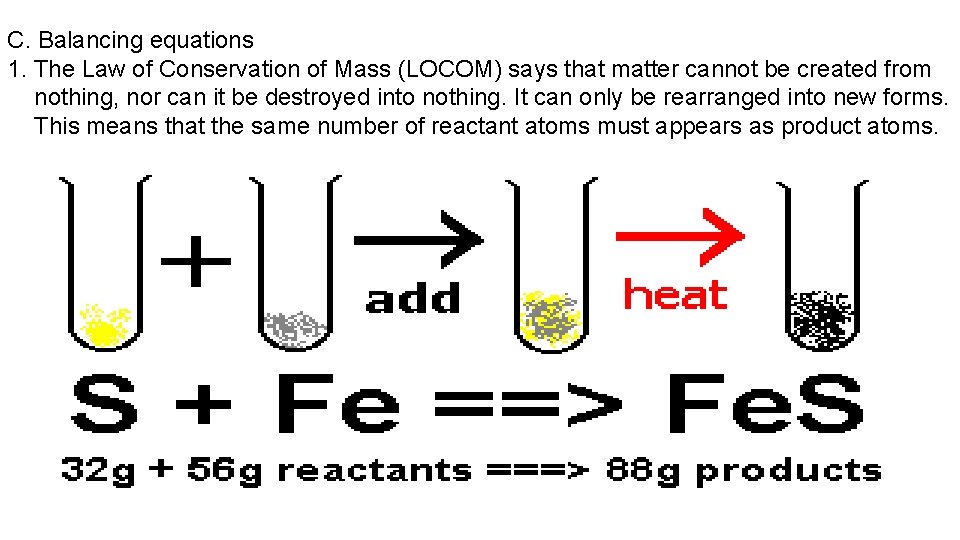
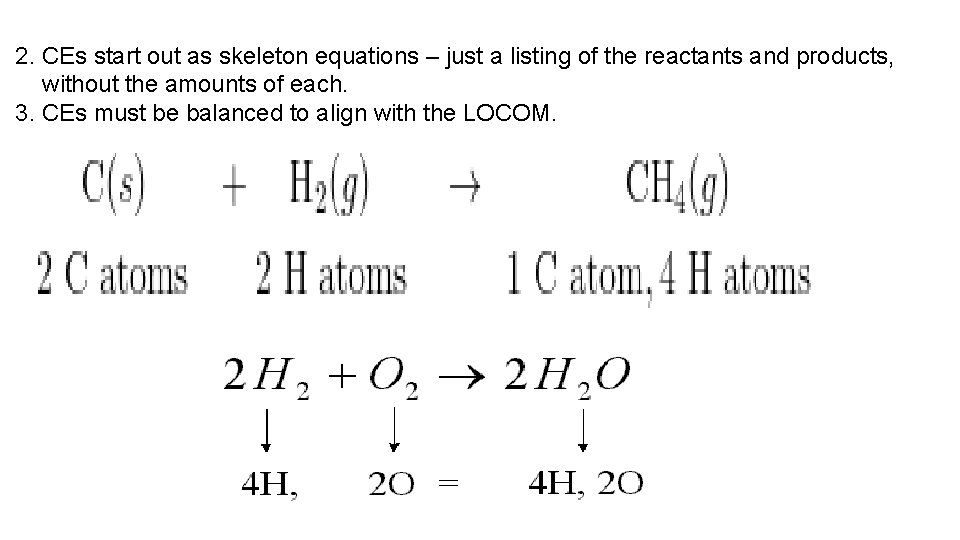
C. Balancing equations 1. The Law of Conservation of Mass (LOCOM) says that matter cannot be created from nothing, nor can it be destroyed into nothing. It can only be rearranged into new forms. This means that the same number of reactant atoms must appears as product atoms.

2. CEs start out as skeleton equations – just a listing of the reactants and products, without the amounts of each. 3. CEs must be balanced to align with the LOCOM.

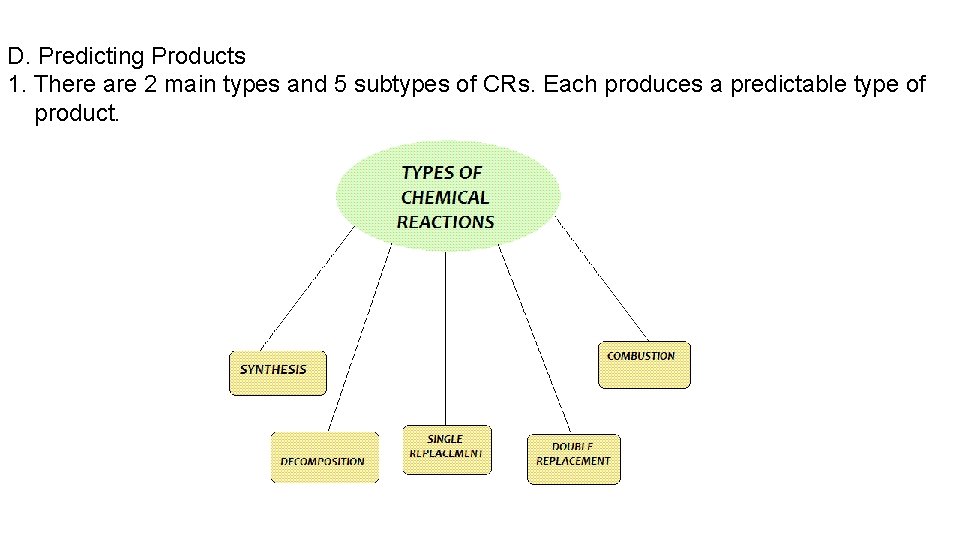
D. Predicting Products 1. There are 2 main types and 5 subtypes of CRs. Each produces a predictable type of product.

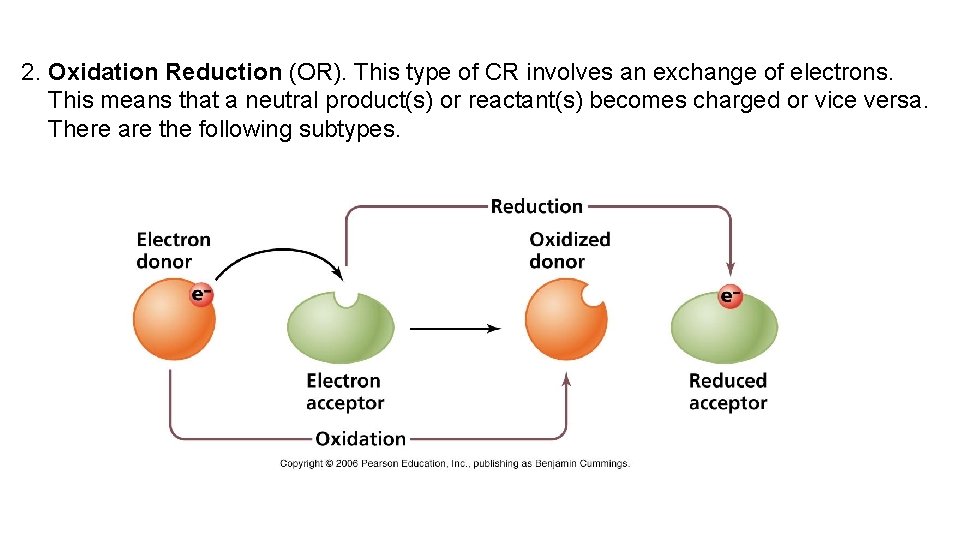
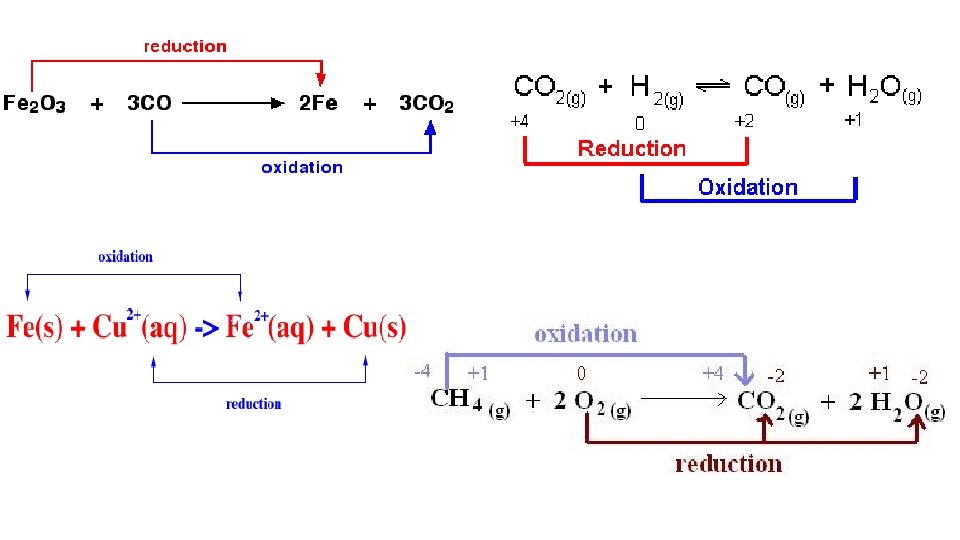
2. Oxidation Reduction (OR). This type of CR involves an exchange of electrons. This means that a neutral product(s) or reactant(s) becomes charged or vice versa. There are the following subtypes.


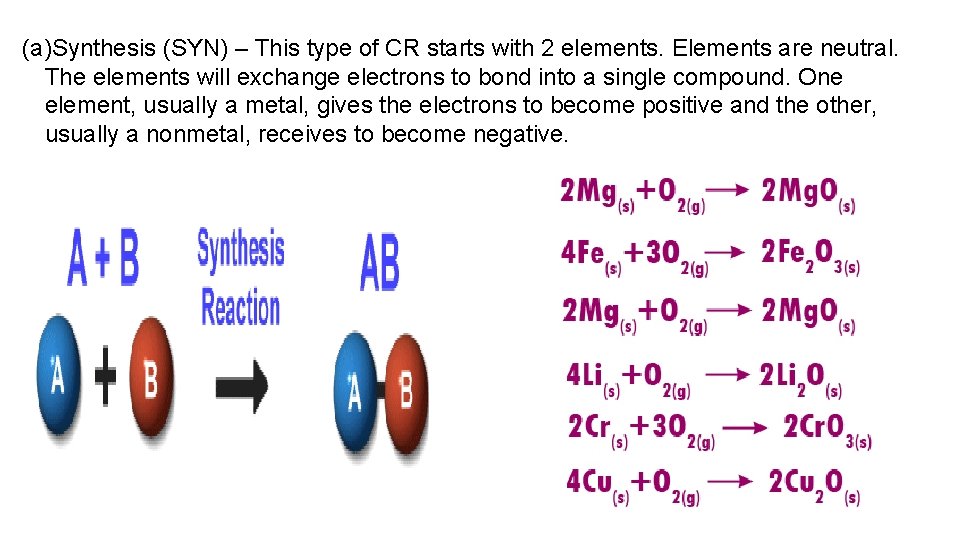
(a)Synthesis (SYN) – This type of CR starts with 2 elements. Elements are neutral. The elements will exchange electrons to bond into a single compound. One element, usually a metal, gives the electrons to become positive and the other, usually a nonmetal, receives to become negative.

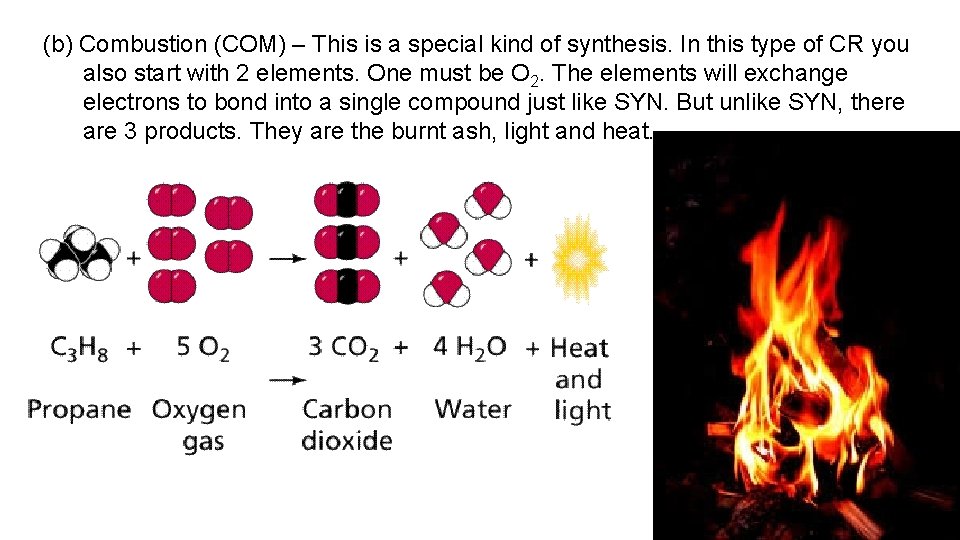
(b) Combustion (COM) – This is a special kind of synthesis. In this type of CR you also start with 2 elements. One must be O 2. The elements will exchange electrons to bond into a single compound just like SYN. But unlike SYN, there are 3 products. They are the burnt ash, light and heat.

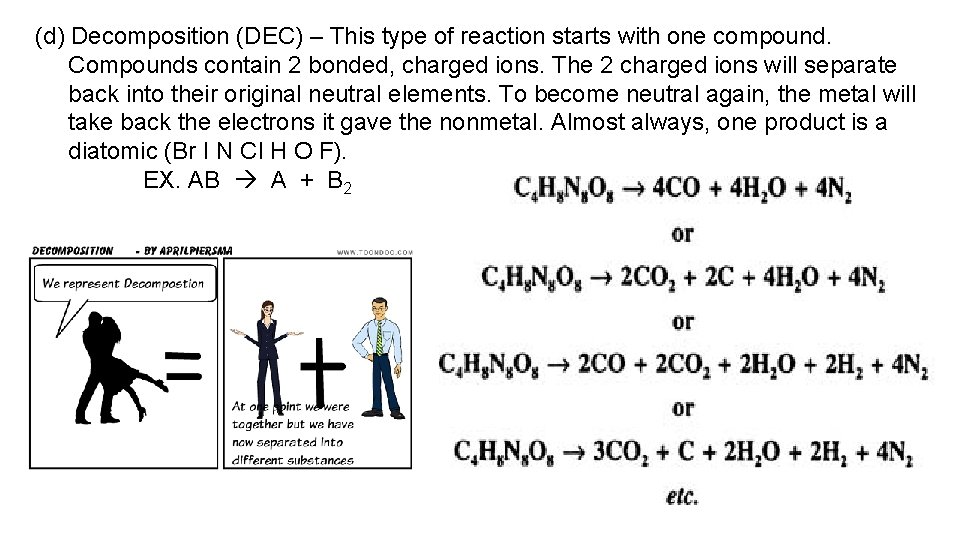
(d) Decomposition (DEC) – This type of reaction starts with one compound. Compounds contain 2 bonded, charged ions. The 2 charged ions will separate back into their original neutral elements. To become neutral again, the metal will take back the electrons it gave the nonmetal. Almost always, one product is a diatomic (Br I N Cl H O F). EX. AB A + B 2

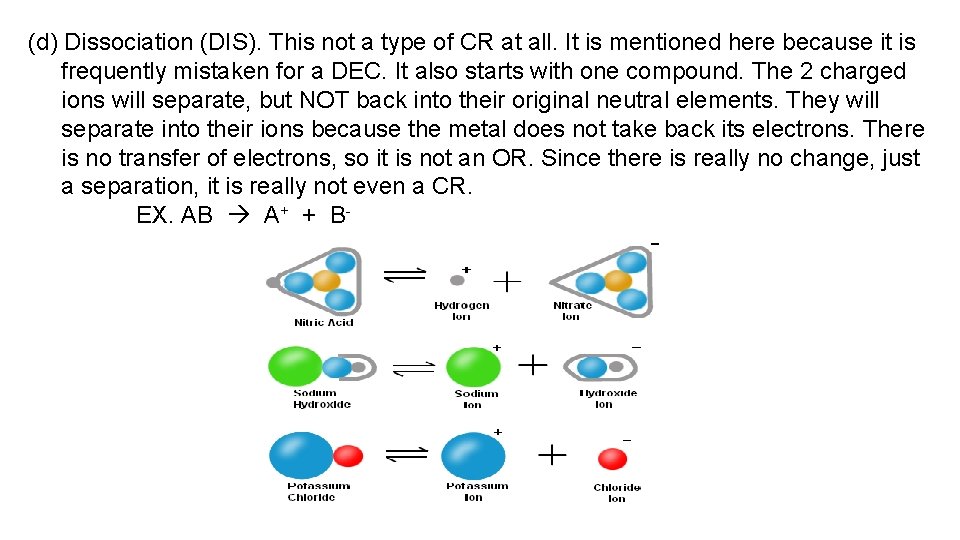
(d) Dissociation (DIS). This not a type of CR at all. It is mentioned here because it is frequently mistaken for a DEC. It also starts with one compound. The 2 charged ions will separate, but NOT back into their original neutral elements. They will separate into their ions because the metal does not take back its electrons. There is no transfer of electrons, so it is not an OR. Since there is really no change, just a separation, it is really not even a CR. EX. AB A+ + B-

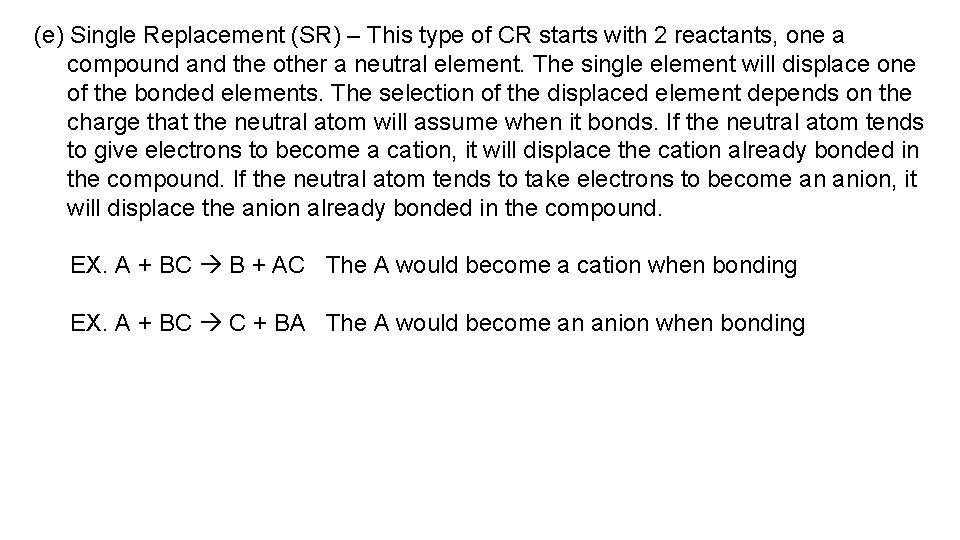
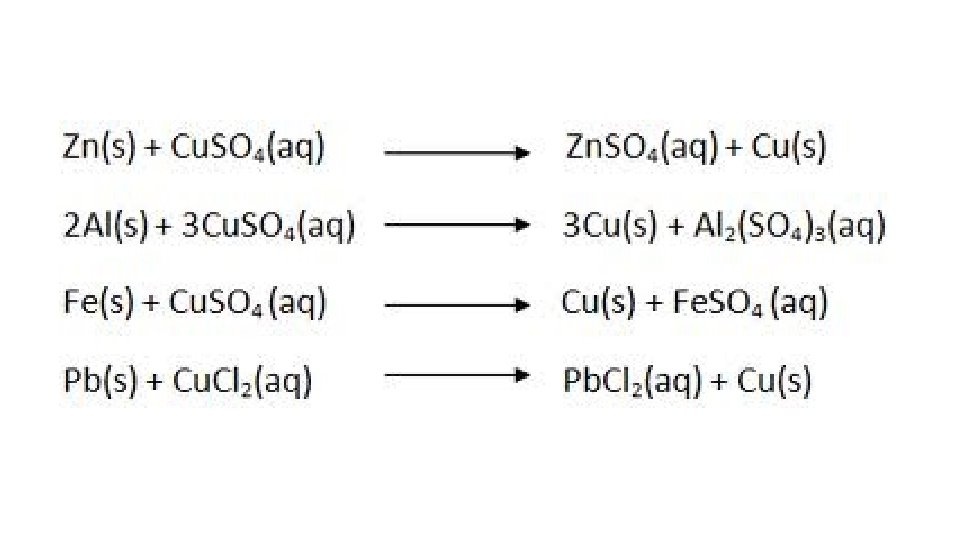
(e) Single Replacement (SR) – This type of CR starts with 2 reactants, one a compound and the other a neutral element. The single element will displace one of the bonded elements. The selection of the displaced element depends on the charge that the neutral atom will assume when it bonds. If the neutral atom tends to give electrons to become a cation, it will displace the cation already bonded in the compound. If the neutral atom tends to take electrons to become an anion, it will displace the anion already bonded in the compound. EX. A + BC B + AC The A would become a cation when bonding EX. A + BC C + BA The A would become an anion when bonding



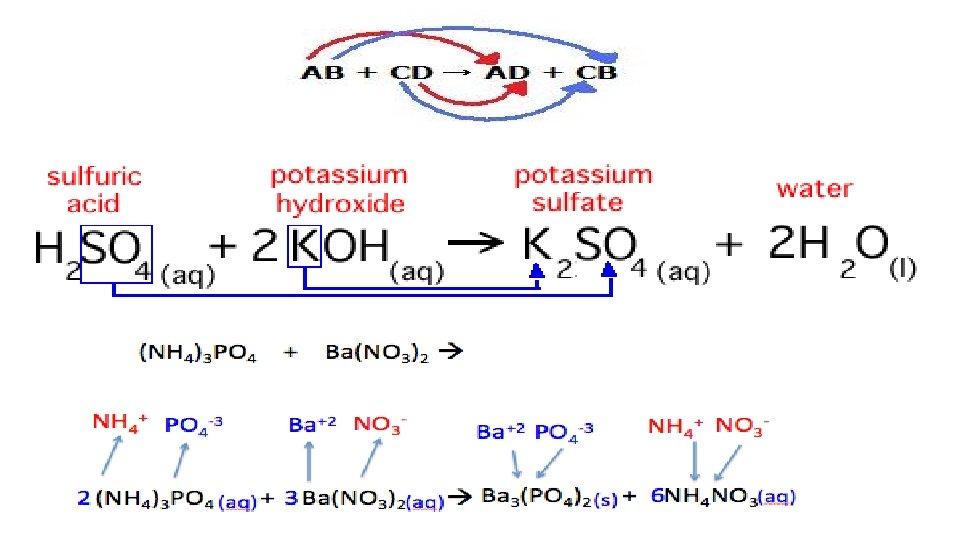
3. Double Replacement (DR) – This type of CR is not an OR. This type of CR does not involve an exchange of electrons. DR starts with 2 reactants; both are compounds. DR ends with 2 products; both are compounds. Compounds contain 2 bonded, charged ions. This means that since both the reactant side and the product side are charged, there was no exchange of electrons. EX. AB + CD AD + CB


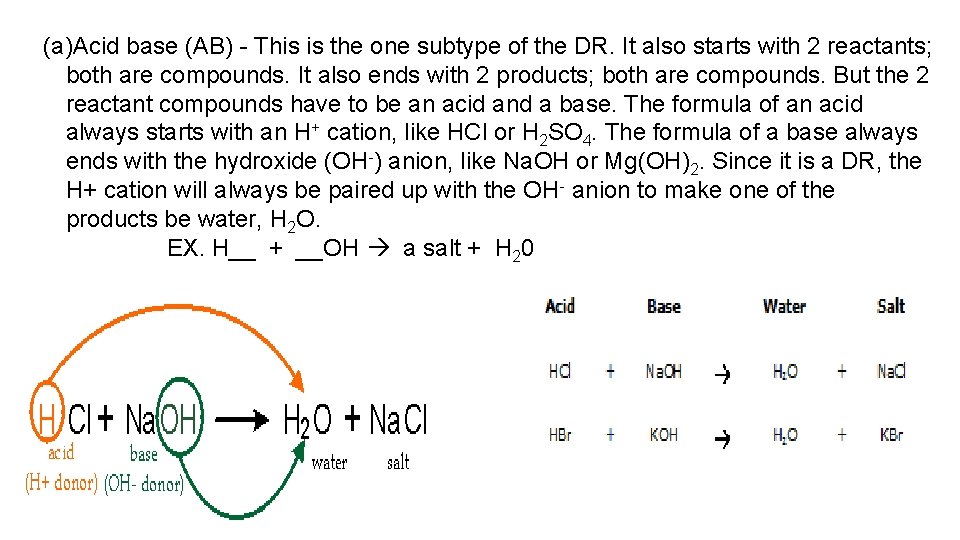
(a)Acid base (AB) - This is the one subtype of the DR. It also starts with 2 reactants; both are compounds. It also ends with 2 products; both are compounds. But the 2 reactant compounds have to be an acid and a base. The formula of an acid always starts with an H+ cation, like HCl or H 2 SO 4. The formula of a base always ends with the hydroxide (OH-) anion, like Na. OH or Mg(OH)2. Since it is a DR, the H+ cation will always be paired up with the OH- anion to make one of the products be water, H 2 O. EX. H__ + __OH a salt + H 20

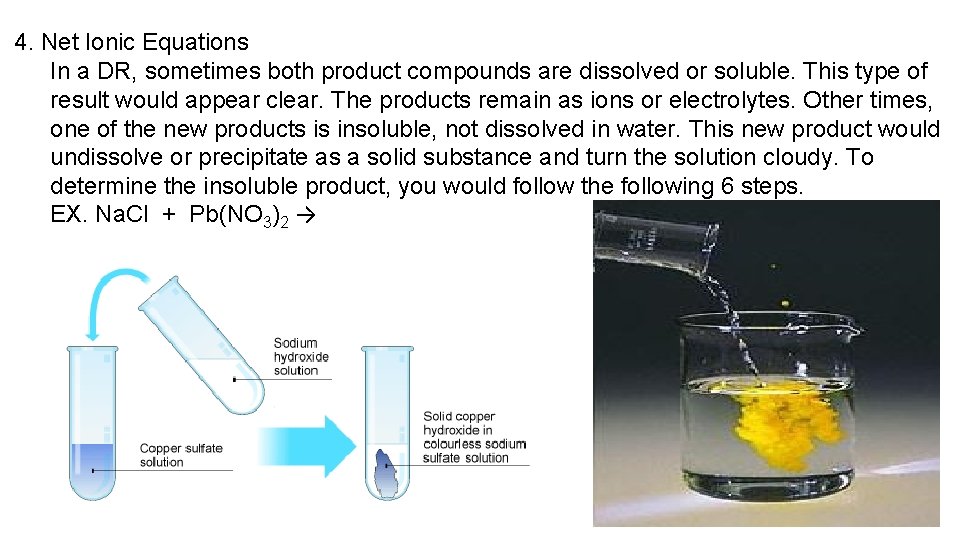
4. Net Ionic Equations In a DR, sometimes both product compounds are dissolved or soluble. This type of result would appear clear. The products remain as ions or electrolytes. Other times, one of the new products is insoluble, not dissolved in water. This new product would undissolve or precipitate as a solid substance and turn the solution cloudy. To determine the insoluble product, you would follow the following 6 steps. EX. Na. Cl + Pb(NO 3)2 →

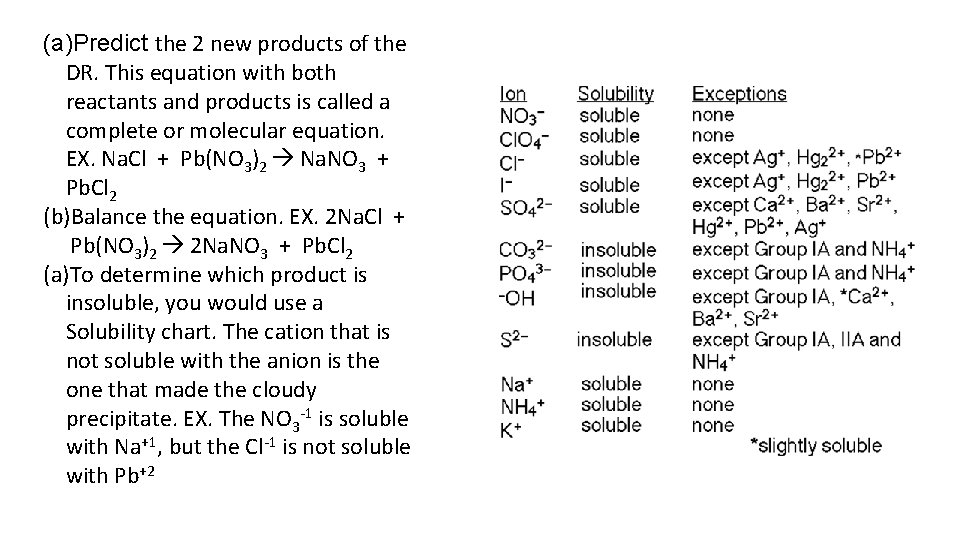
(a)Predict the 2 new products of the DR. This equation with both reactants and products is called a complete or molecular equation. EX. Na. Cl + Pb(NO 3)2 Na. NO 3 + Pb. Cl 2 (b)Balance the equation. EX. 2 Na. Cl + Pb(NO 3)2 2 Na. NO 3 + Pb. Cl 2 (a)To determine which product is insoluble, you would use a Solubility chart. The cation that is not soluble with the anion is the one that made the cloudy precipitate. EX. The NO 3 -1 is soluble with Na+1, but the Cl-1 is not soluble with Pb+2

(d) Write the net ionic equation by starting with the 2 ions that created your insoluble compound (Pb. Cl 2). Record them as the reactants WITH their charge. DO NOT Br I N Cl H O F any reactant. Then finish the equation with the correct formula of the insoluble product. Ex. Pb+2 + Cl-1 Pb. Cl 2 (e) Assign symbols. The product has a (s) and the 2 reactants are always (aq). Ex. Pb+2 (aq) + Cl-1 (aq) Pb. Cl 2 (s) (f) Balance it. EX. Pb+2 (aq) + 2 Cl-1 (aq) Pb. Cl 2 (s)

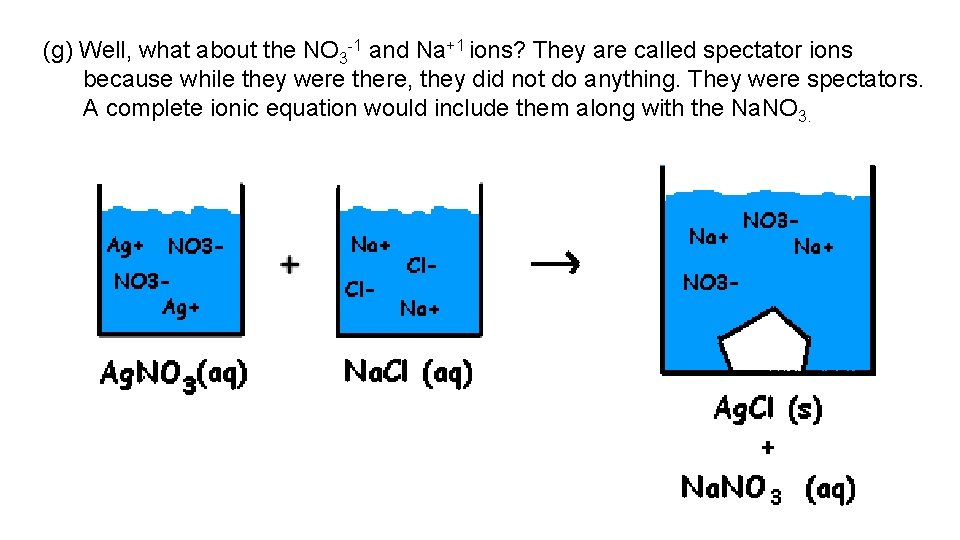
(g) Well, what about the NO 3 -1 and Na+1 ions? They are called spectator ions because while they were there, they did not do anything. They were spectators. A complete ionic equation would include them along with the Na. NO 3.

 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Section 1 chemical changes
Section 1 chemical changes Half redox reaction
Half redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Predicting products of chemical reactions
Predicting products of chemical reactions What are active metals
What are active metals Mass relationships in chemical reactions
Mass relationships in chemical reactions Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chemical equation worksheet
Chemical equation worksheet Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Mnvii
Mnvii Solvent in chemical reactions
Solvent in chemical reactions Types of reactions chemistry
Types of reactions chemistry Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Atoms rearranging
Atoms rearranging Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Type of chemical reactions
Type of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the














































