Redox Reactions Categorizing Chemical Reactions All chemical reactions


- Slides: 12

Redox Reactions

Categorizing Chemical Reactions �All chemical reactions involve something moving, so we can categorize the types of reactions we just learned to help us predict the products in a reaction! Moving electrons = synthesis, decomposition, and single displacement Moving ions = double displacement Moving protons = acid/base reaction (we’ll get into this in a later unit) Combustion is actually a combination of these

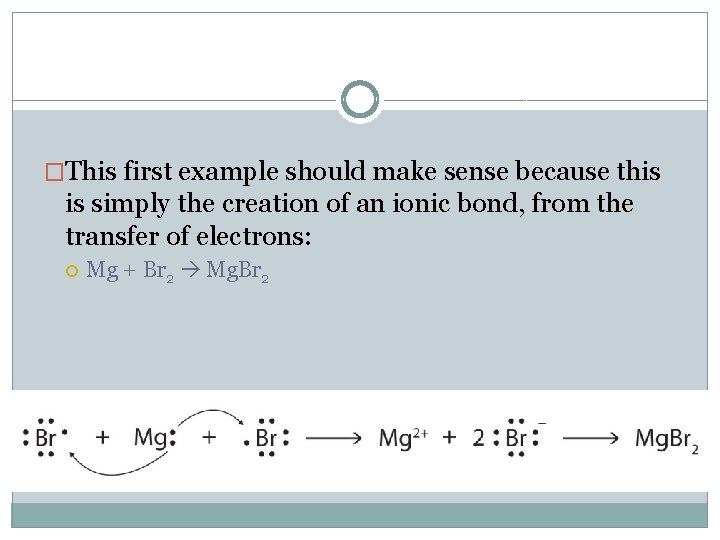
�This first example should make sense because this is simply the creation of an ionic bond, from the transfer of electrons: Mg + Br 2 Mg. Br 2

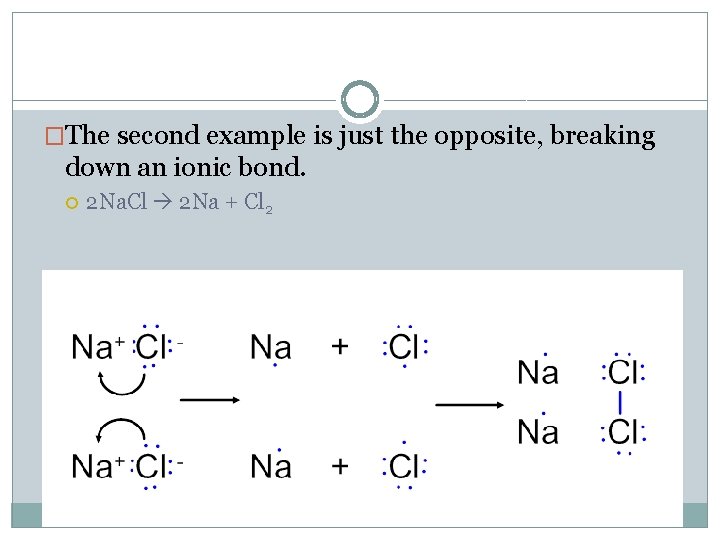
�The second example is just the opposite, breaking down an ionic bond. 2 Na. Cl 2 Na + Cl 2

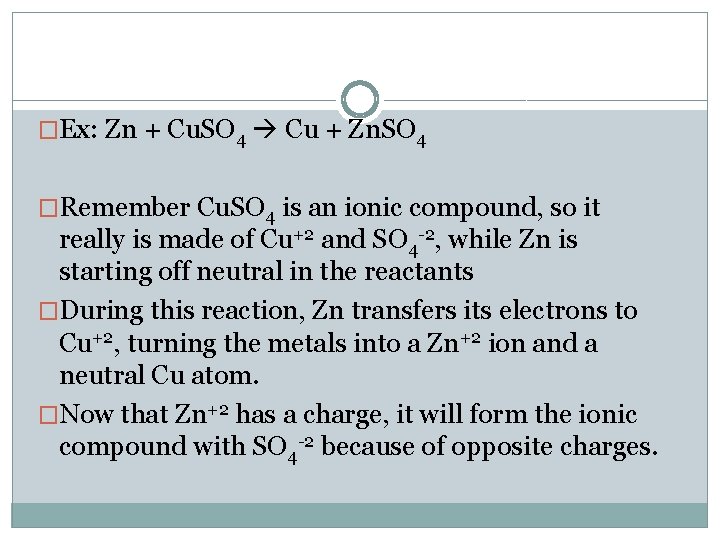
�Ex: Zn + Cu. SO 4 Cu + Zn. SO 4 �Remember Cu. SO 4 is an ionic compound, so it really is made of Cu+2 and SO 4 -2, while Zn is starting off neutral in the reactants �During this reaction, Zn transfers its electrons to Cu+2, turning the metals into a Zn+2 ion and a neutral Cu atom. �Now that Zn+2 has a charge, it will form the ionic compound with SO 4 -2 because of opposite charges.

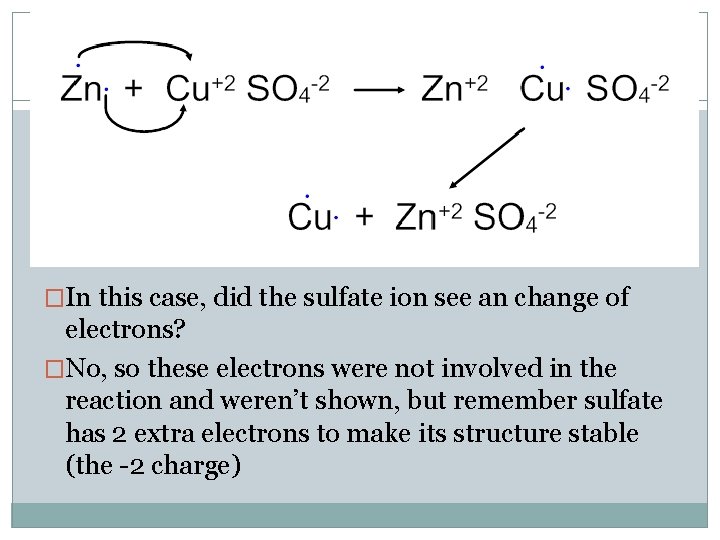
�In this case, did the sulfate ion see an change of electrons? �No, so these electrons were not involved in the reaction and weren’t shown, but remember sulfate has 2 extra electrons to make its structure stable (the -2 charge)

Reduction and Oxidation �In these reactions, one atom or element is reduced and the other is oxidized So you’ll often hear these called Redox reactions �A substance that is reduced has gained electrons and therefore reduced its charge �A substance that is oxidized has lost electrons

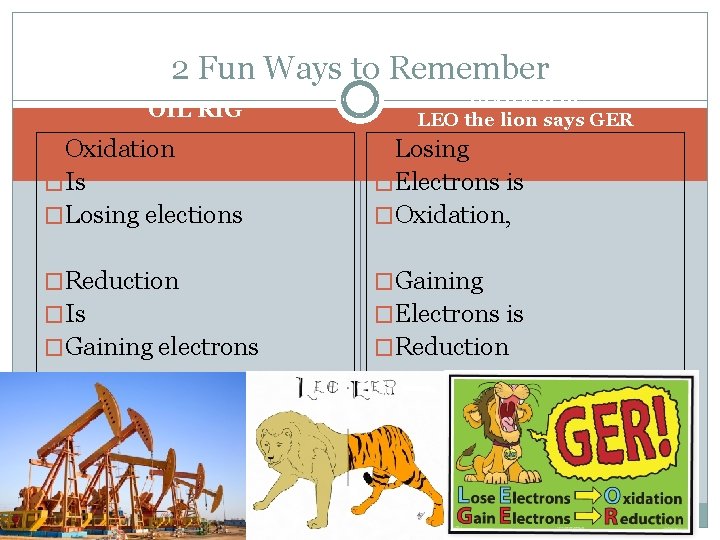
2 Fun Ways to Remember OIL RIG LEOGER or LEO the lion says GER �Oxidation �Losing �Is �Electrons is �Losing elections �Oxidation, �Reduction �Gaining �Is �Electrons is �Gaining electrons �Reduction

And now, the tricky part… �A substance that has been oxidized is called a reducing AGENT because it caused the other substance to take their electrons �A substance that has been reduced is called an oxidizing AGENT because it caused the other substance to give up its own electrons

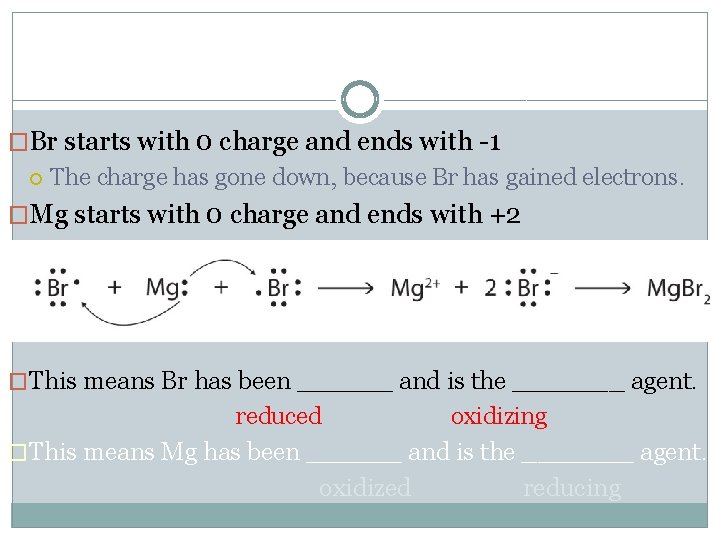
�Br starts with 0 charge and ends with -1 The charge has gone down, because Br has gained electrons. �Mg starts with 0 charge and ends with +2 The charge has gone up, because Mg has lost electrons. �This means Br has been ______ and is the _______ agent. reduced oxidizing �This means Mg has been ______ and is the _______ agent. oxidized reducing

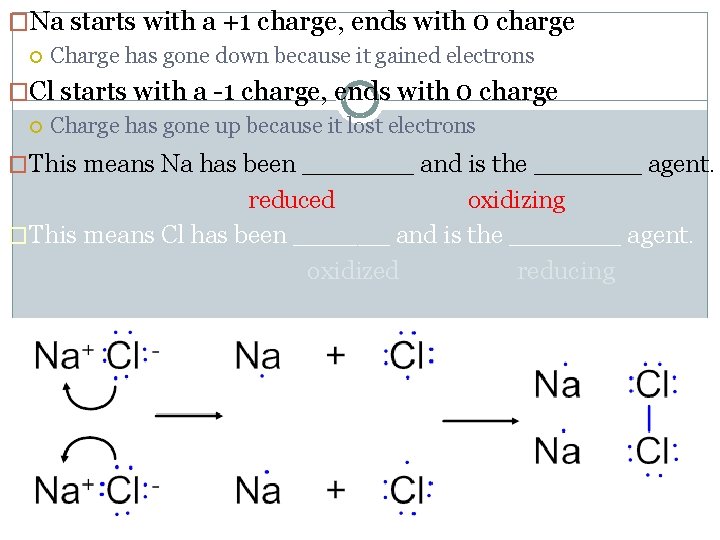
�Na starts with a +1 charge, ends with 0 charge Charge has gone down because it gained electrons �Cl starts with a -1 charge, ends with 0 charge Charge has gone up because it lost electrons �This means Na has been _______ and is the ______ agent. reduced oxidizing �This means Cl has been ______ and is the _______ agent. oxidized reducing

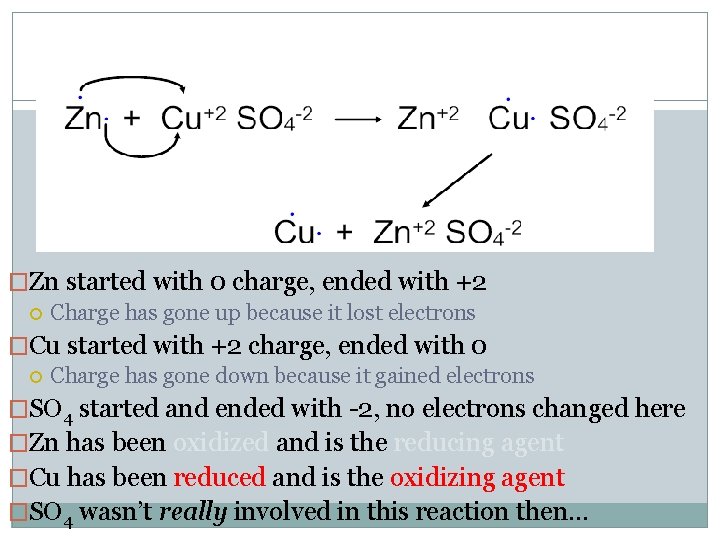
�Zn started with 0 charge, ended with +2 Charge has gone up because it lost electrons �Cu started with +2 charge, ended with 0 Charge has gone down because it gained electrons �SO 4 started and ended with -2, no electrons changed here �Zn has been oxidized and is the reducing agent �Cu has been reduced and is the oxidizing agent �SO 4 wasn’t really involved in this reaction then…
 Reduction half reaction
Reduction half reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Different types of redox reactions
Different types of redox reactions Types of reactions
Types of reactions Categorizing different types of memory
Categorizing different types of memory Objective-based categorizing projects is possible
Objective-based categorizing projects is possible Unit 2 materials technology
Unit 2 materials technology Categorizing equations
Categorizing equations Land biomes lesson outline answers
Land biomes lesson outline answers Oil rig oxidation
Oil rig oxidation Balancing aqueous solutions
Balancing aqueous solutions Half reduction reaction
Half reduction reaction