Types of Chemical Reactions Types of Reactions There









- Slides: 24

Types of Chemical Reactions

Types of Reactions There are six types of chemical reactions we will talk about: 1. 2. 3. 4. 5. 6. Synthesis reactions (syn) Decomposition reactions (decomp) Single replacement reactions (SR) Double replacement reactions (DR) Combustion reactions (Comb) Oxidation-Reduction reactions (Redox)

Synthesis Reactions (AKA Combination or Addition) Two substances (generally elements) combine and form a compound. reactant + reactant 1 product A + B AB 2 H 2 + O 2 2 H 2 O C + O 2 CO 2

Synthesis Reactions

Decomposition Reactions Compound breaks up into the elements or into a few simpler compounds 1 Reactant Product + Product AB A + B 2 H 2 O 2 H 2 + O 2 2 Hg. O 2 Hg + O 2

Decomposition Reactions

Single Replacement or Displacement Reactions One element replaces another in a compound metal can replace a metal (+) OR nonmetal can replace a nonmetal (-) element + compound product + product A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!)


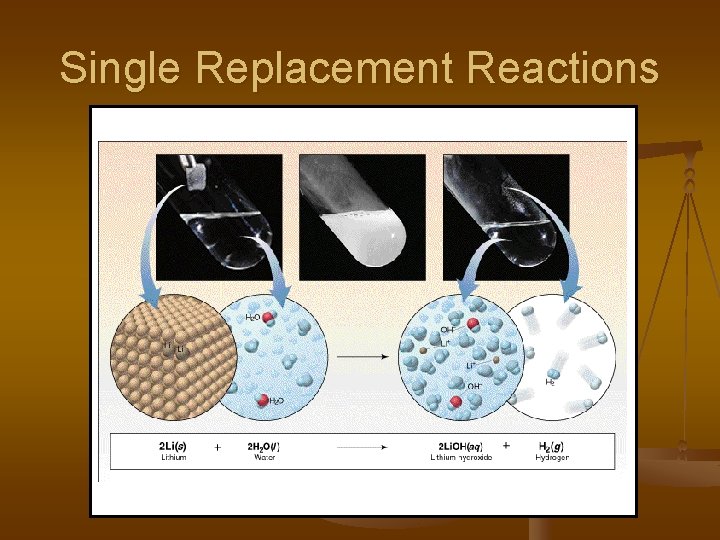
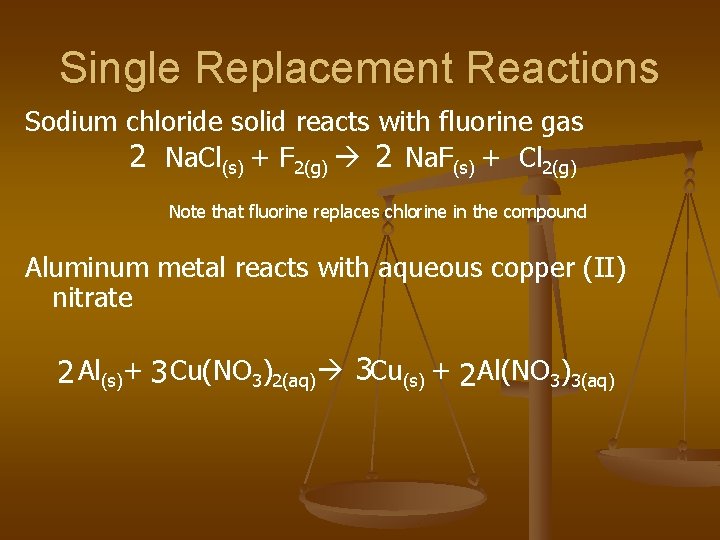
Single Replacement Reactions

Single Replacement Reactions Sodium chloride solid reacts with fluorine gas 2 Na. Cl(s) + F 2(g) 2 Na. F(s) + Cl 2(g) Note that fluorine replaces chlorine in the compound Aluminum metal reacts with aqueous copper (II) nitrate 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s) + 2 Al(NO 3)3(aq)

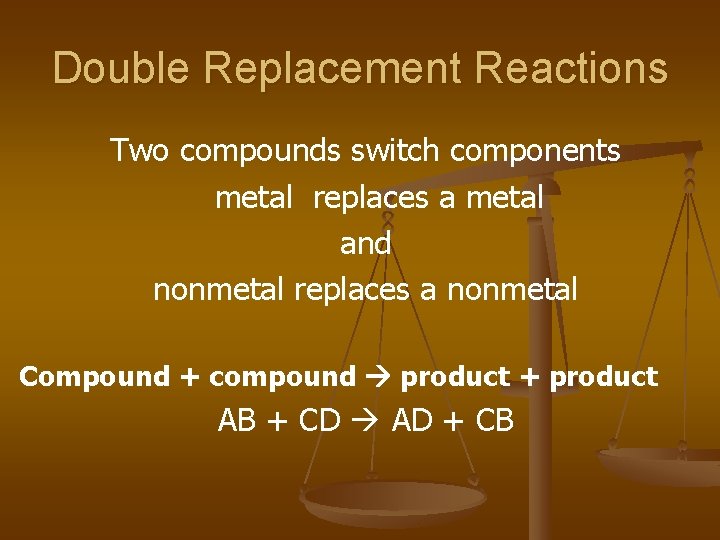
Double Replacement Reactions Two compounds switch components metal replaces a metal and nonmetal replaces a nonmetal Compound + compound product + product AB + CD AD + CB


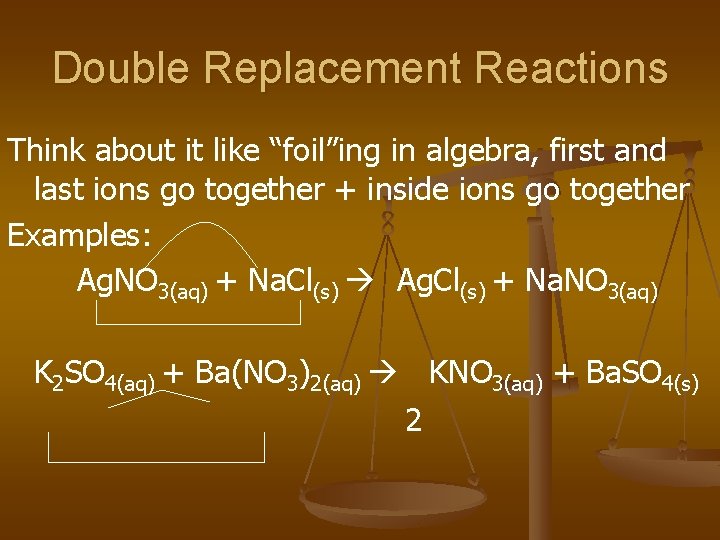
Double Replacement Reactions Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Examples: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) K 2 SO 4(aq) + Ba(NO 3)2(aq) KNO 3(aq) + Ba. SO 4(s) 2

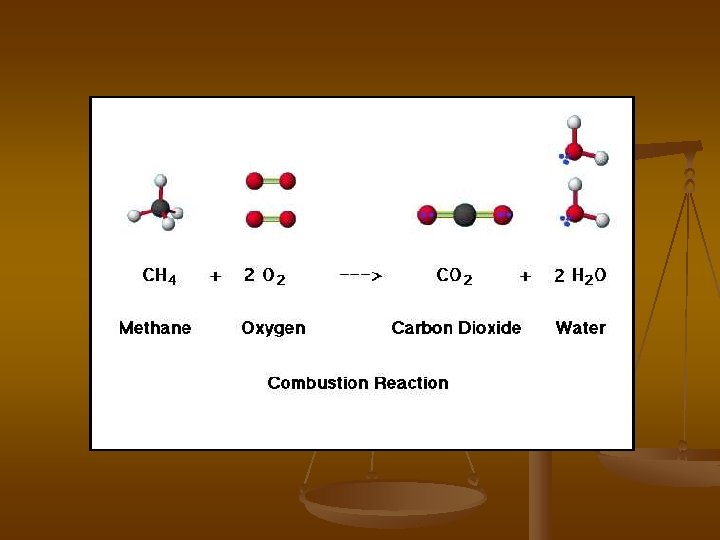
Combustion Reactions Hydrocarbon reacts with oxygen gas to form carbon dioxide and water and energy Cy. Hx + O 2 → CO 2 + H 2 O + E (heat) Methane- Natural Gas CH 4


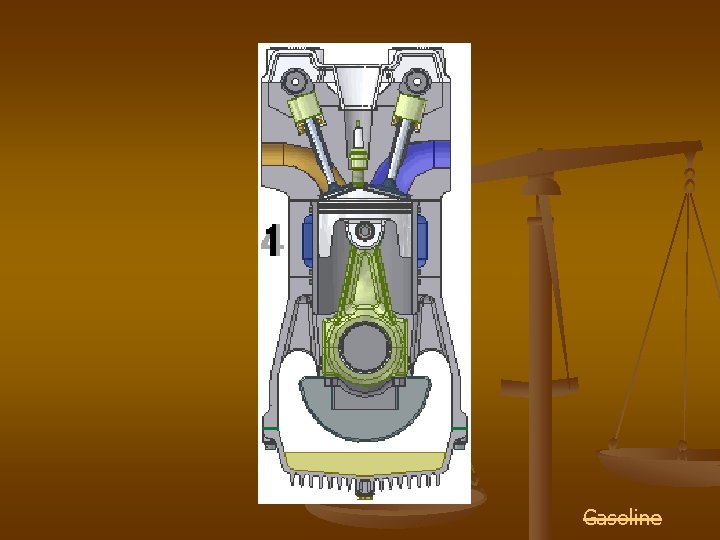
Combustion Reactions A car has an internal combustion engine

Gasoline

By Products of Fuel Combustion CO NOx SOx

Incomplete Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.

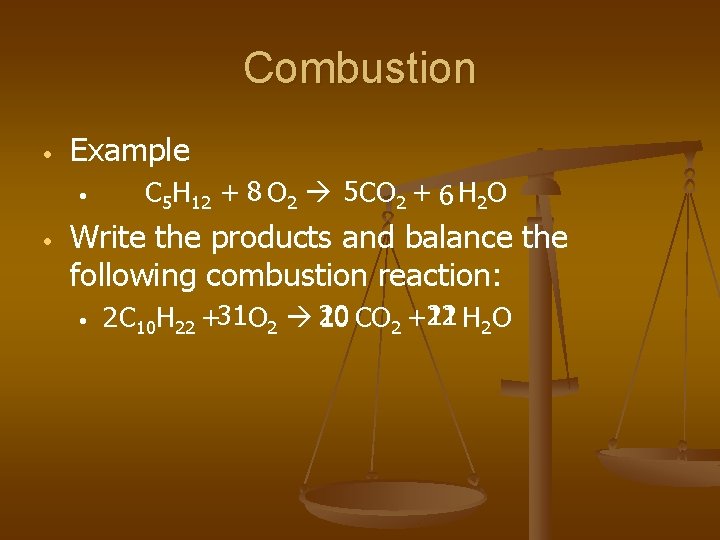
Combustion • Example • • C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O Write the products and balance the following combustion reaction: • 11 H 2 O 2 C 10 H 22 +31 O 2 20 10 CO 2 +22

Oxidation-Reduction (Redox) Many of the 5 types of reactions are also oxidation-reduction reactions Oxidation: Loss of electrons (RA) Reduction: Gain of electrons (OA)

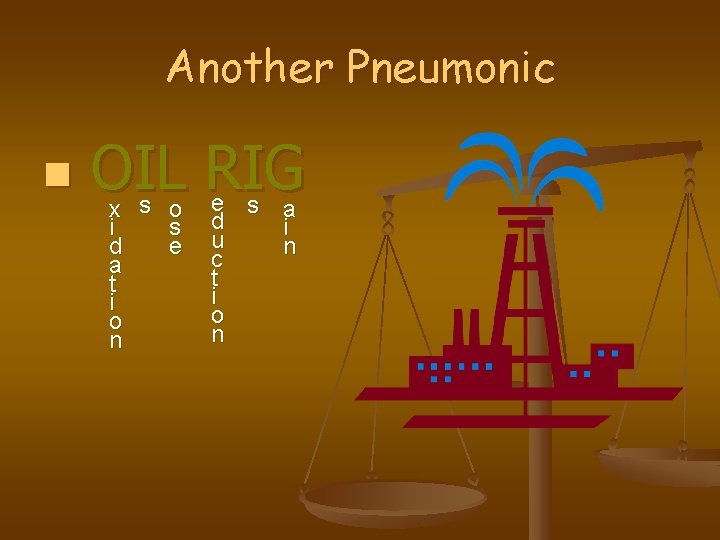
You can’t have one… without the other! If an element (metal) is losing e-, then another element (nonmetal) has to gain e. There has to be conservation of e- lost and gained in a reaction LEO the lion says GER! o s e GER! l e c t r o n s x i d a t i o n a i n l e c t r o n s e d u c t i o n

Another Pneumonic n OIL RIG x s o i s d e a t i o n e s a d i u n c t i o n

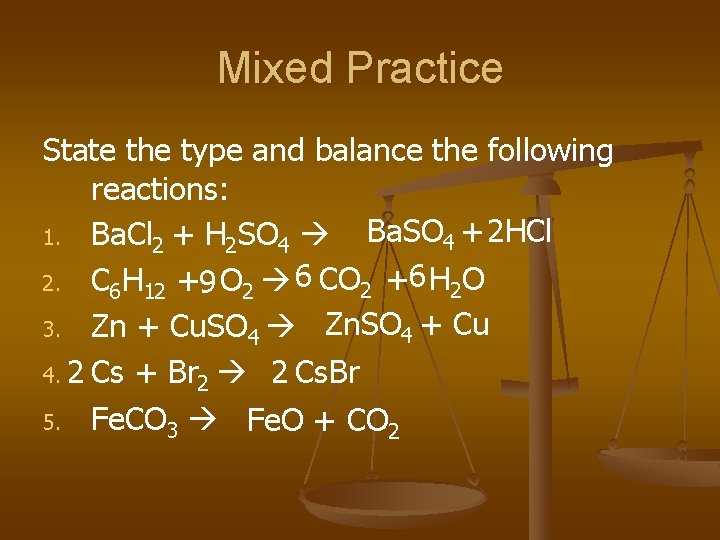
Mixed Practice State the type and balance the following reactions: Ba. SO 4 + 2 HCl 1. Ba. Cl 2 + H 2 SO 4 2. C 6 H 12 +9 O 2 6 CO 2 +6 H 2 O 3. Zn + Cu. SO 4 Zn. SO 4 + Cu 4. 2 Cs + Br 2 2 Cs. Br 5. Fe. CO 3 Fe. O + CO 2
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of chemical reactions redox
Types of chemical reactions redox Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef What are the five general types of chemical reactions
What are the five general types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Redox reactions examples
Redox reactions examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Stoichiometry mole island diagram
Stoichiometry mole island diagram I intro
I intro Predicting products of chemical reactions
Predicting products of chemical reactions