Energy and Chemical Reactions Types of Reactions Energy
- Slides: 14

Energy and Chemical Reactions

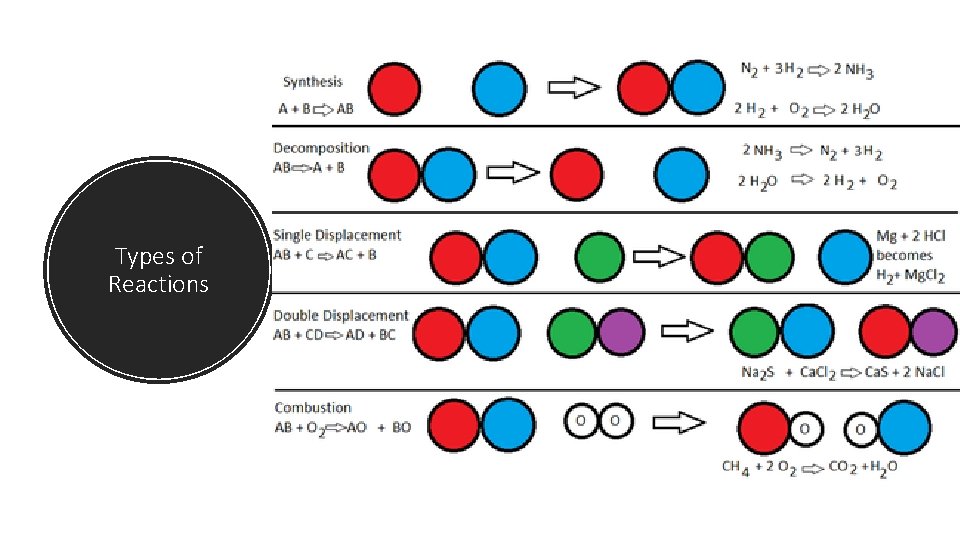
Types of Reactions

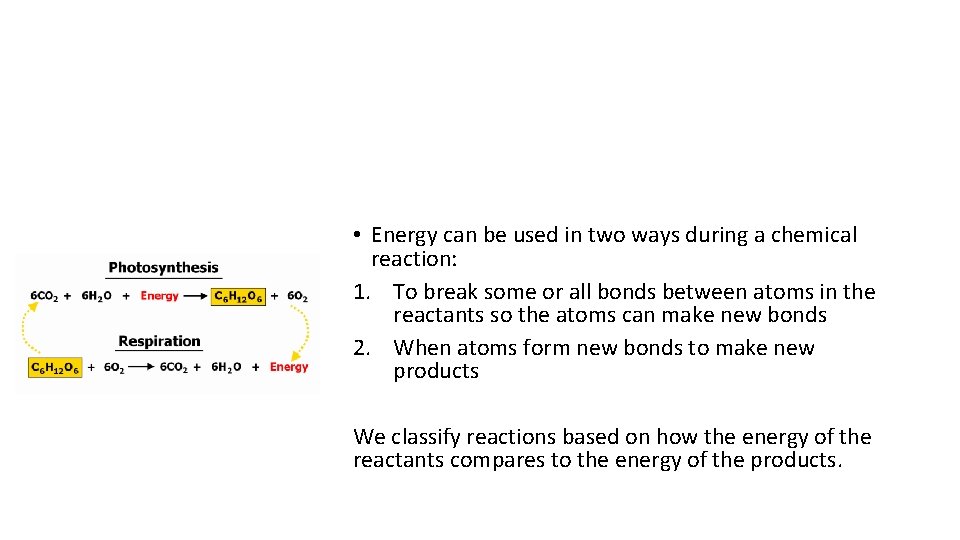
Energy in Chemical Reactions • Energy can be used in two ways during a chemical reaction: 1. To break some or all bonds between atoms in the reactants so the atoms can make new bonds 2. When atoms form new bonds to make new products We classify reactions based on how the energy of the reactants compares to the energy of the products.

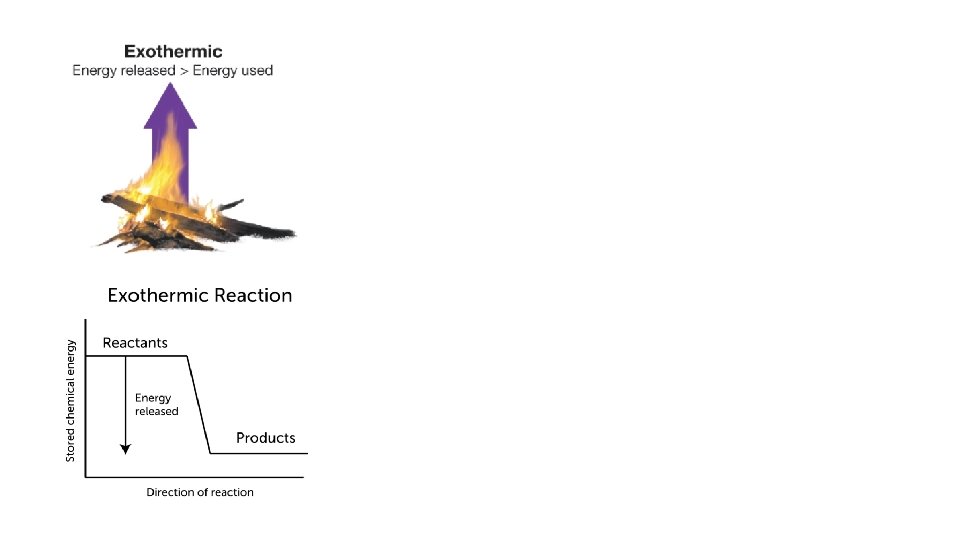
Exothermic Reactions • Exothermic reactions release more energy than it takes to break the old bonds • Examples of exothermic reactions include explosions, burning fossil fuels, and cellular respiration

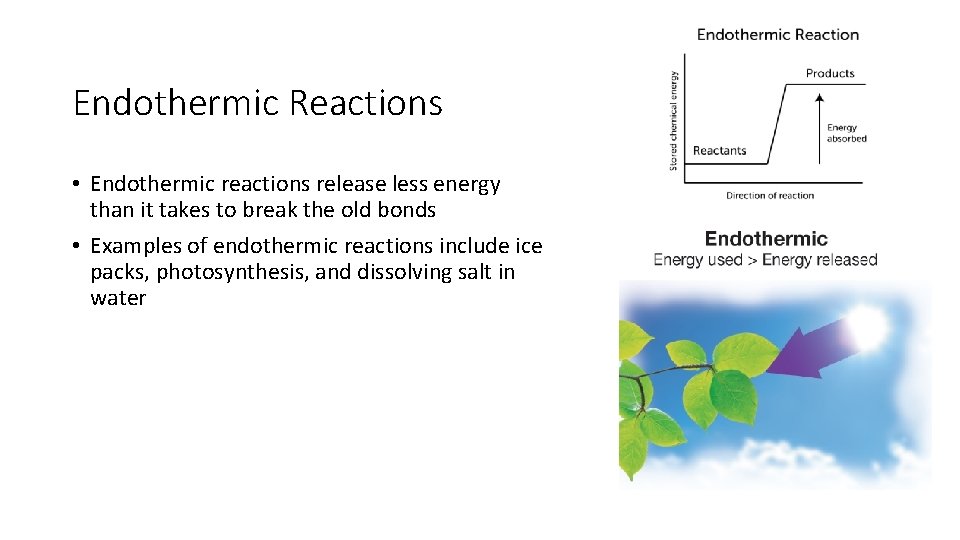
Endothermic Reactions • Endothermic reactions release less energy than it takes to break the old bonds • Examples of endothermic reactions include ice packs, photosynthesis, and dissolving salt in water

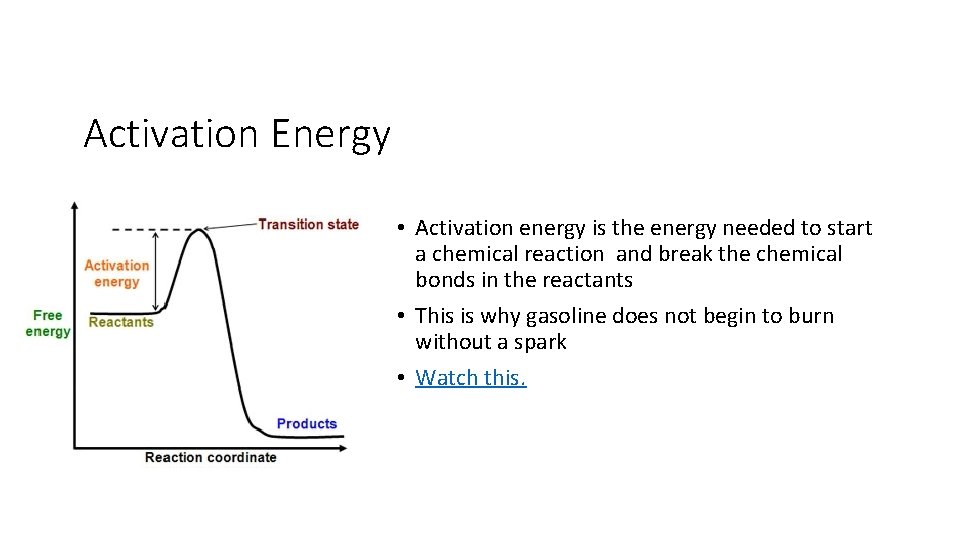
Activation Energy • Activation energy is the energy needed to start a chemical reaction and break the chemical bonds in the reactants • This is why gasoline does not begin to burn without a spark • Watch this.

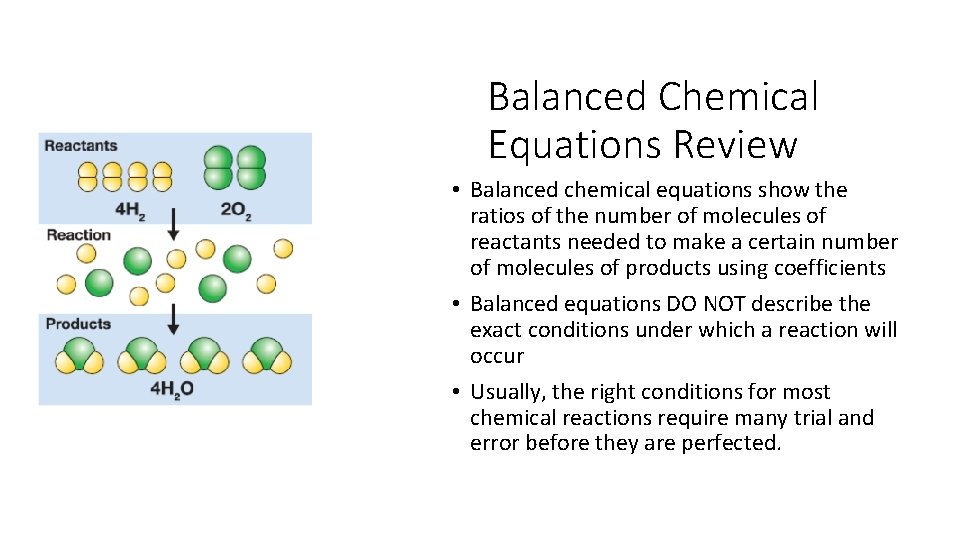
Balanced Chemical Equations Review • Balanced chemical equations show the ratios of the number of molecules of reactants needed to make a certain number of molecules of products using coefficients • Balanced equations DO NOT describe the exact conditions under which a reaction will occur • Usually, the right conditions for most chemical reactions require many trial and error before they are perfected.

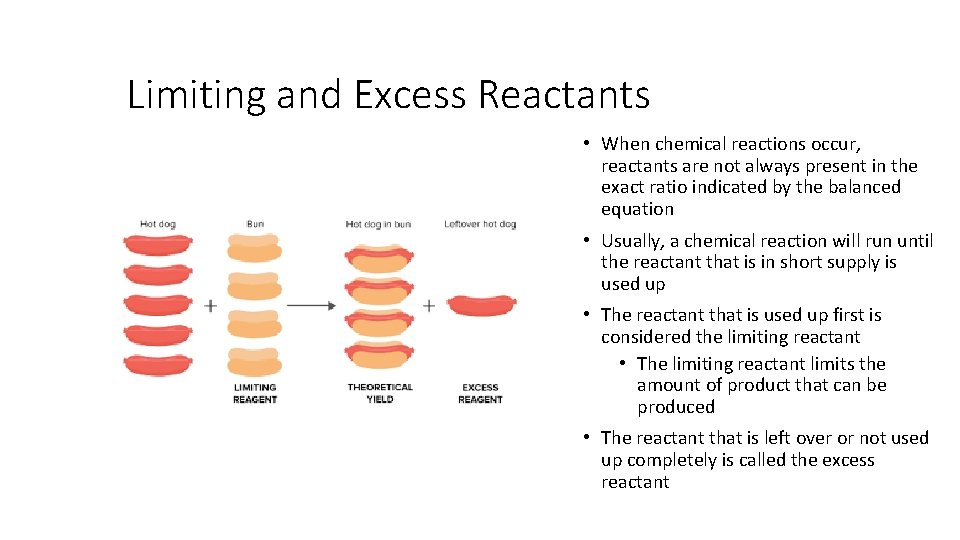
Limiting and Excess Reactants • When chemical reactions occur, reactants are not always present in the exact ratio indicated by the balanced equation • Usually, a chemical reaction will run until the reactant that is in short supply is used up • The reactant that is used up first is considered the limiting reactant • The limiting reactant limits the amount of product that can be produced • The reactant that is left over or not used up completely is called the excess reactant

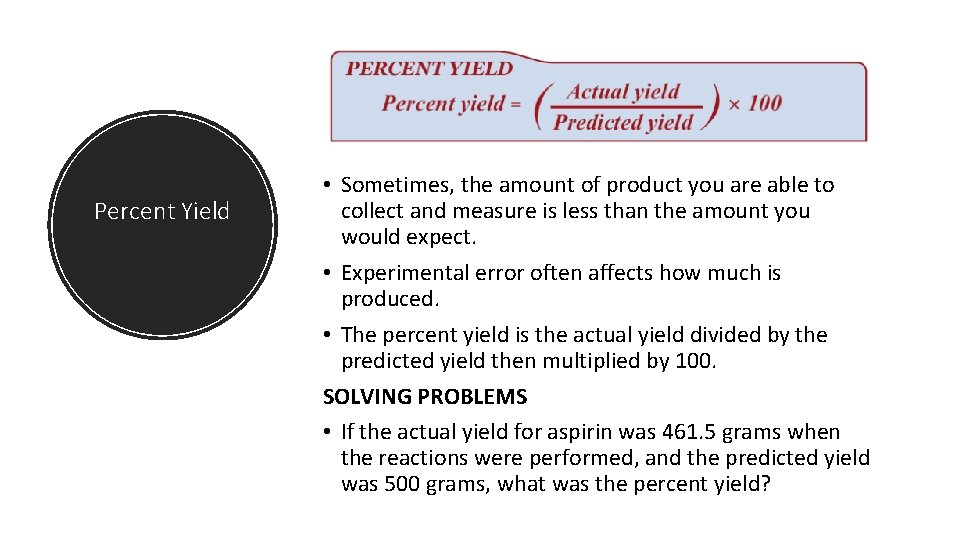
Percent Yield • Sometimes, the amount of product you are able to collect and measure is less than the amount you would expect. • Experimental error often affects how much is produced. • The percent yield is the actual yield divided by the predicted yield then multiplied by 100. SOLVING PROBLEMS • If the actual yield for aspirin was 461. 5 grams when the reactions were performed, and the predicted yield was 500 grams, what was the percent yield?

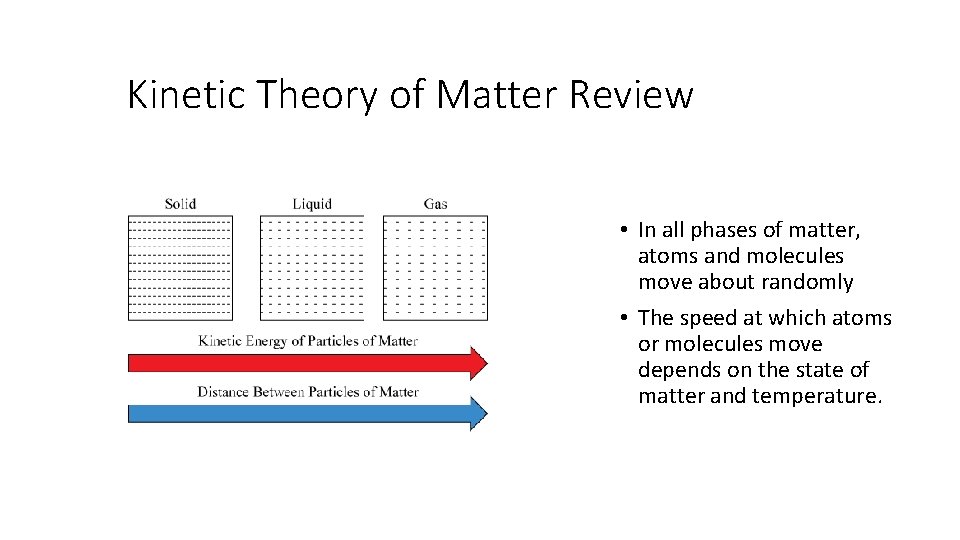
Kinetic Theory of Matter Review • In all phases of matter, atoms and molecules move about randomly • The speed at which atoms or molecules move depends on the state of matter and temperature.

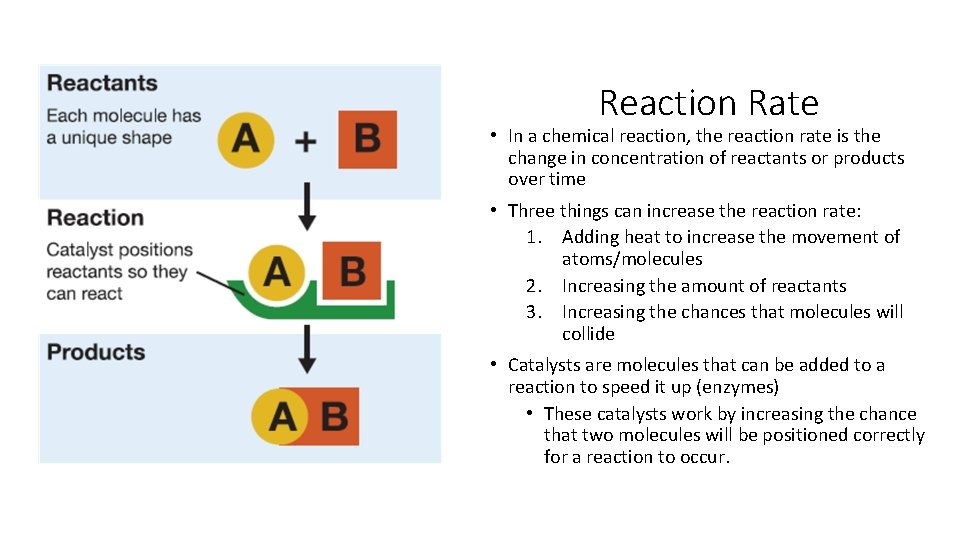
Reaction Rate • In a chemical reaction, the reaction rate is the change in concentration of reactants or products over time • Three things can increase the reaction rate: 1. Adding heat to increase the movement of atoms/molecules 2. Increasing the amount of reactants 3. Increasing the chances that molecules will collide • Catalysts are molecules that can be added to a reaction to speed it up (enzymes) • These catalysts work by increasing the chance that two molecules will be positioned correctly for a reaction to occur.

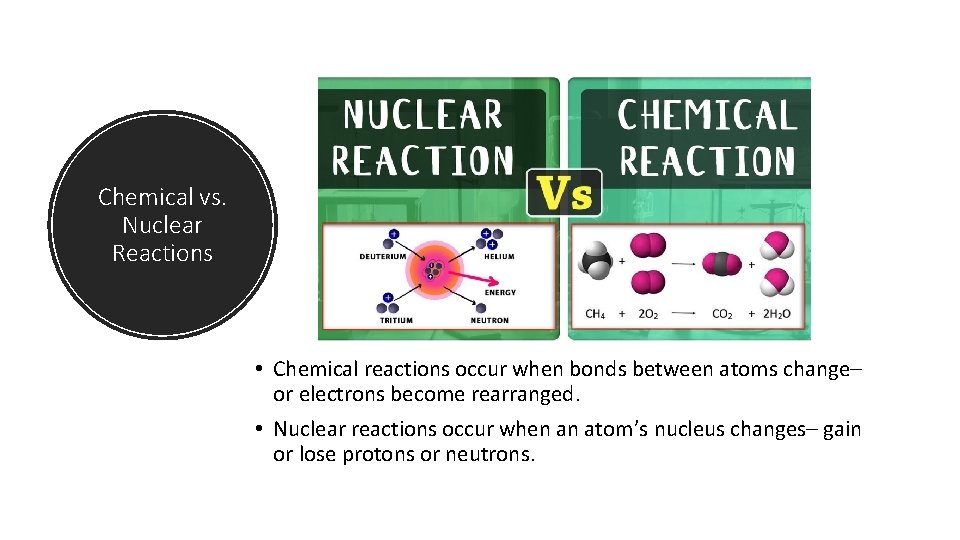
Chemical vs. Nuclear Reactions • Chemical reactions occur when bonds between atoms change– or electrons become rearranged. • Nuclear reactions occur when an atom’s nucleus changes– gain or lose protons or neutrons.

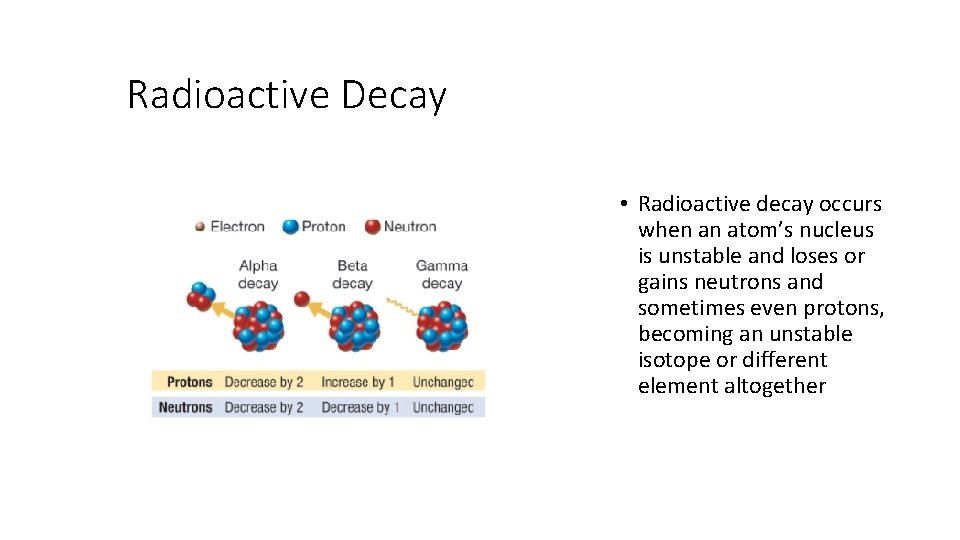
Radioactive Decay • Radioactive decay occurs when an atom’s nucleus is unstable and loses or gains neutrons and sometimes even protons, becoming an unstable isotope or different element altogether

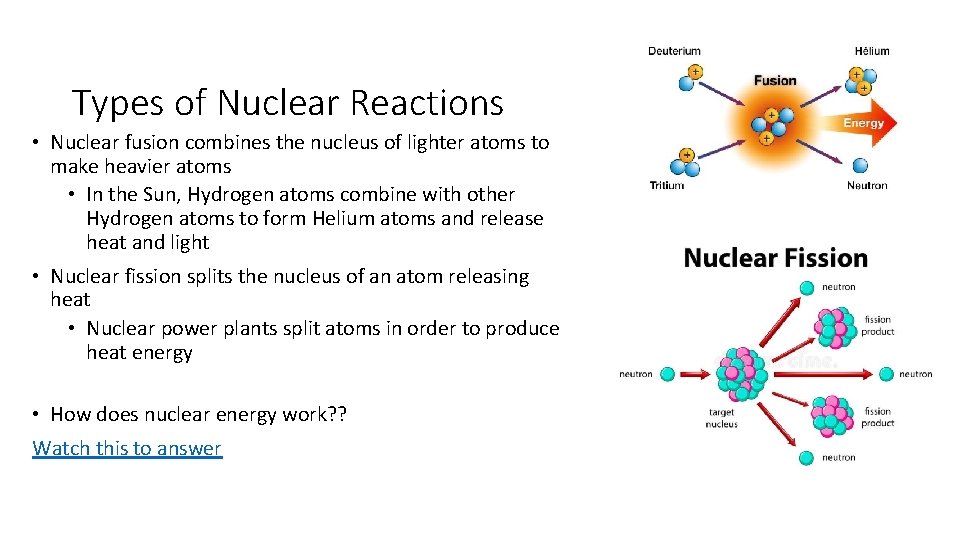
Types of Nuclear Reactions • Nuclear fusion combines the nucleus of lighter atoms to make heavier atoms • In the Sun, Hydrogen atoms combine with other Hydrogen atoms to form Helium atoms and release heat and light • Nuclear fission splits the nucleus of an atom releasing heat • Nuclear power plants split atoms in order to produce heat energy • How does nuclear energy work? ? Watch this to answer
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Balancing redox reactions
Balancing redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions Chemical reaction types
Chemical reaction types 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are the five chemical changes
What are the five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions


























