14 1 Chemical Reactions Chemical Reactions Chemical reaction








- Slides: 21

14. 1 Chemical Reactions

Chemical Reactions Chemical reaction: the process of breaking chemical bonds and reforming new bonds to make new substances

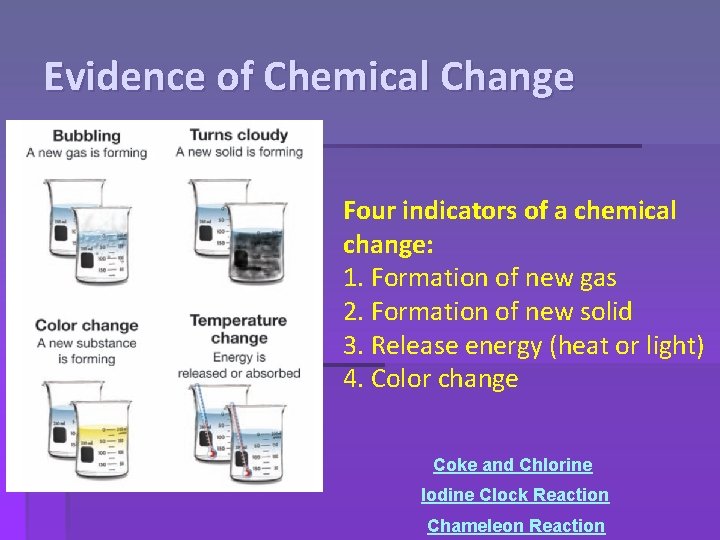
Evidence of Chemical Change Four indicators of a chemical change: 1. Formation of new gas 2. Formation of new solid 3. Release energy (heat or light) 4. Color change Coke and Chlorine Iodine Clock Reaction Chameleon Reaction

Reactants and Products Reactant: starting substance(s) Product: new substance(s) formed

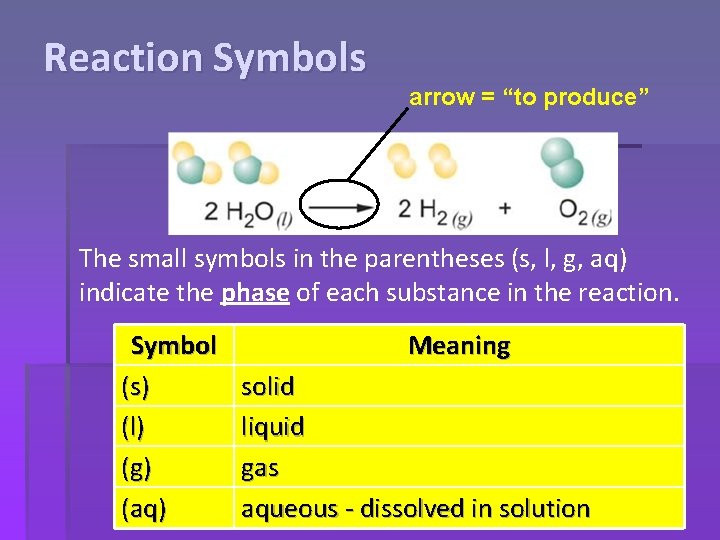
Reaction Symbols arrow = “to produce” The small symbols in the parentheses (s, l, g, aq) indicate the phase of each substance in the reaction. Symbol (s) (l) (g) (aq) Meaning solid liquid gas aqueous - dissolved in solution

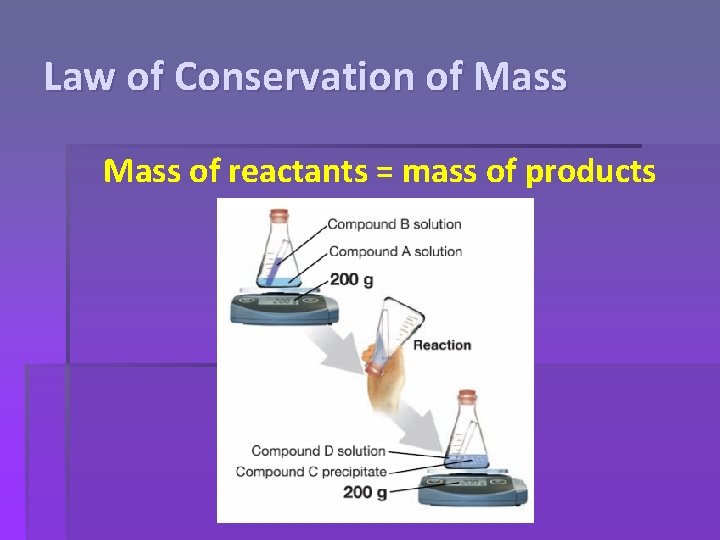
Law of Conservation of Mass: Mass cannot be created or destroyed, only rearranged.

Law of Conservation of Mass of reactants = mass of products

Example § 5. 0 g of magnesium (Mg) burns in 1. 0 g of oxygen (O 2). How many grams of magnesium oxide (Mg. O) should be produced? Mg + O 2 Mg. O

Check-In § 10. 2 g of Lithium Fluoride (Li. F) and an unknown amount of sodium chloride (Na. Cl) react to form 13. 5 g of Lithium chloride (Li. Cl) and 21. 2 g of Sodium fluoride (Na. F). How many grams of sodium chloride reacted? Li. F + Na. Cl Li. Cl + Na. F

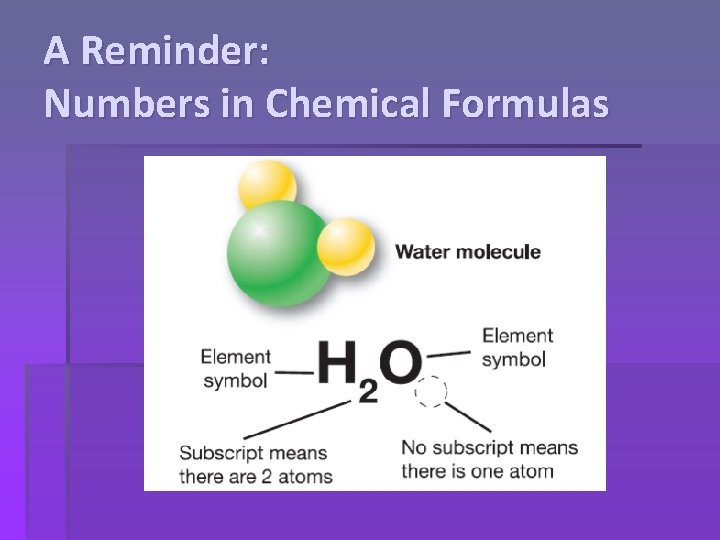
A Reminder: Numbers in Chemical Formulas

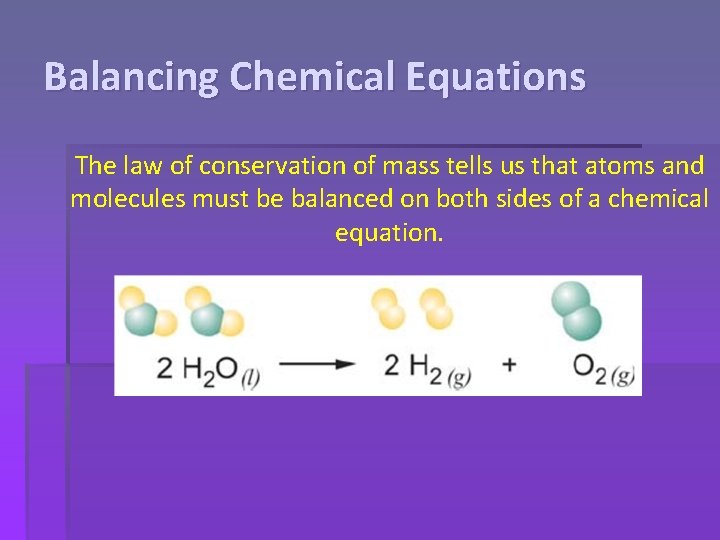
Balancing Chemical Equations The law of conservation of mass tells us that atoms and molecules must be balanced on both sides of a chemical equation.

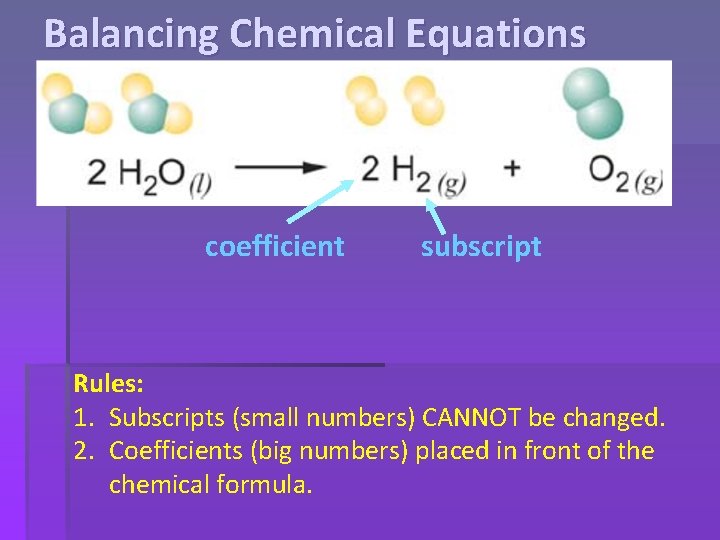
Balancing Chemical Equations coefficient subscript Rules: 1. Subscripts (small numbers) CANNOT be changed. 2. Coefficients (big numbers) placed in front of the chemical formula.

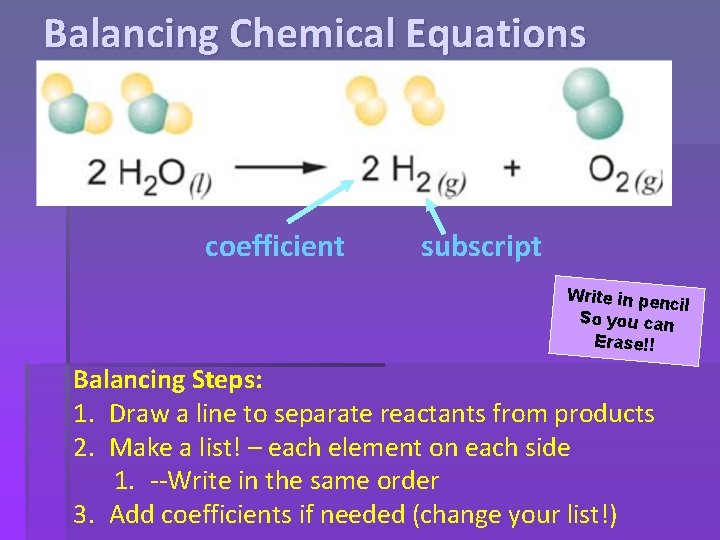
Balancing Chemical Equations coefficient subscript Write in penc il So you can Erase!! Balancing Steps: 1. Draw a line to separate reactants from products 2. Make a list! – each element on each side 1. --Write in the same order 3. Add coefficients if needed (change your list!)

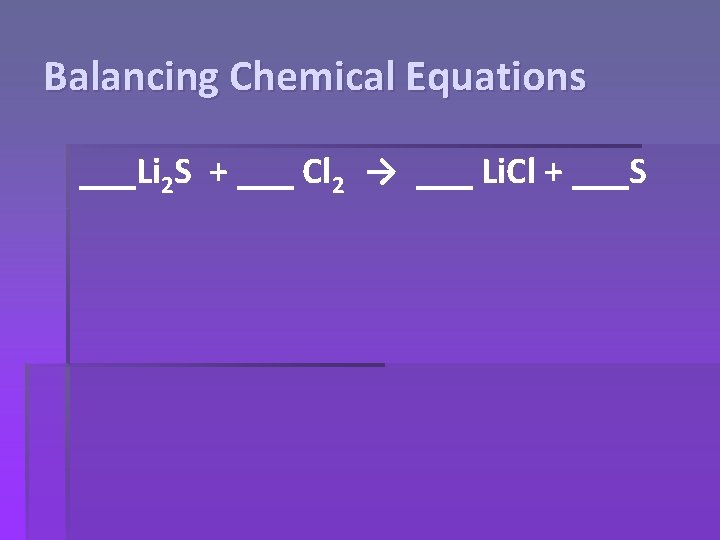
Balancing Chemical Equations ___Li 2 S + ___ Cl 2 → ___ Li. Cl + ___S

Balancing Chemical Equations ___Mg + ___ O 2 → ___ Mg. O

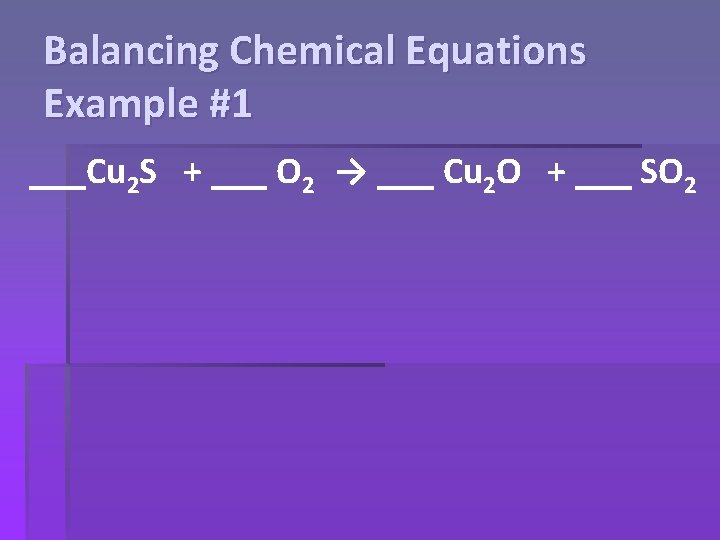
Balancing Chemical Equations Example #1 ___Cu 2 S + ___ O 2 → ___ Cu 2 O + ___ SO 2

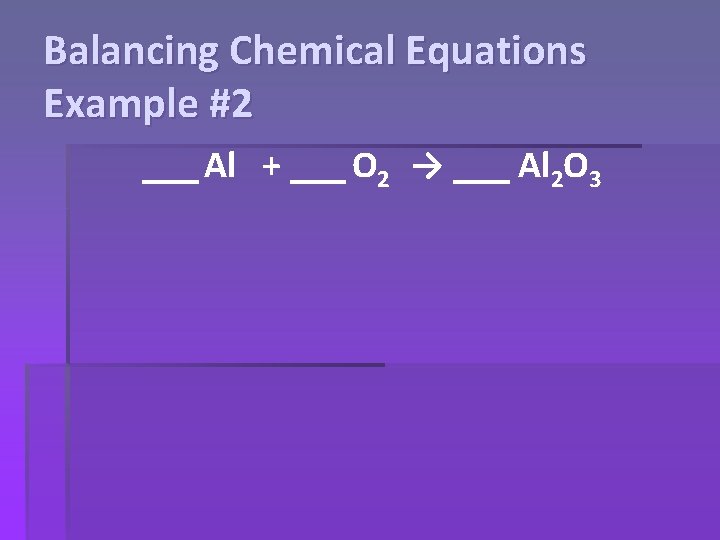
Balancing Chemical Equations Example #2 ___ Al + ___ O 2 → ___ Al 2 O 3

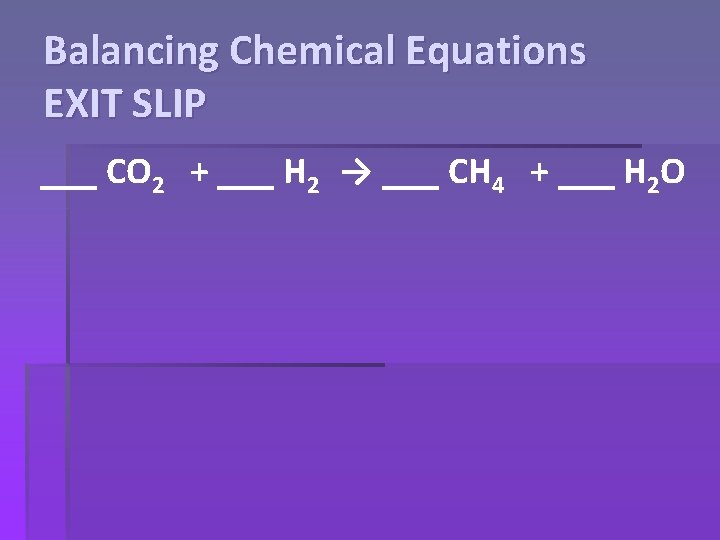
Balancing Chemical Equations EXIT SLIP ___ CO 2 + ___ H 2 → ___ CH 4 + ___ H 2 O

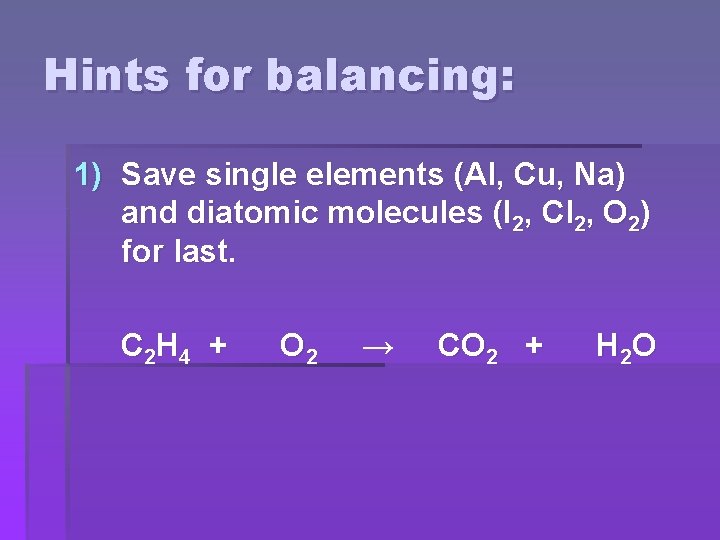
Hints for balancing: 1) Save single elements (Al, Cu, Na) and diatomic molecules (I 2, Cl 2, O 2) for last. C 2 H 4 + O 2 → CO 2 + H 2 O

Hints for balancing: 2) Find Common Multiples KCl. O 3 → KCl + O 2

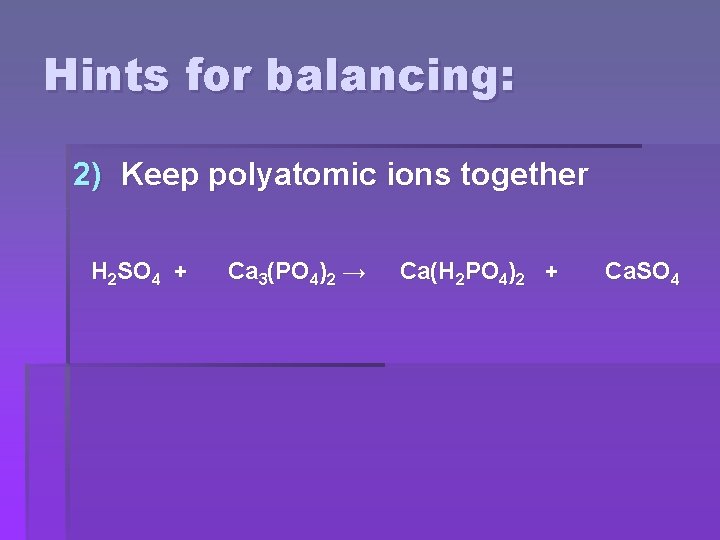
Hints for balancing: 2) Keep polyatomic ions together H 2 SO 4 + Ca 3(PO 4)2 → Ca(H 2 PO 4)2 + Ca. SO 4