Chemical Reactions vs Nuclear Reactions Chemical Reactions vs

- Slides: 10

Chemical Reactions vs. Nuclear Reactions

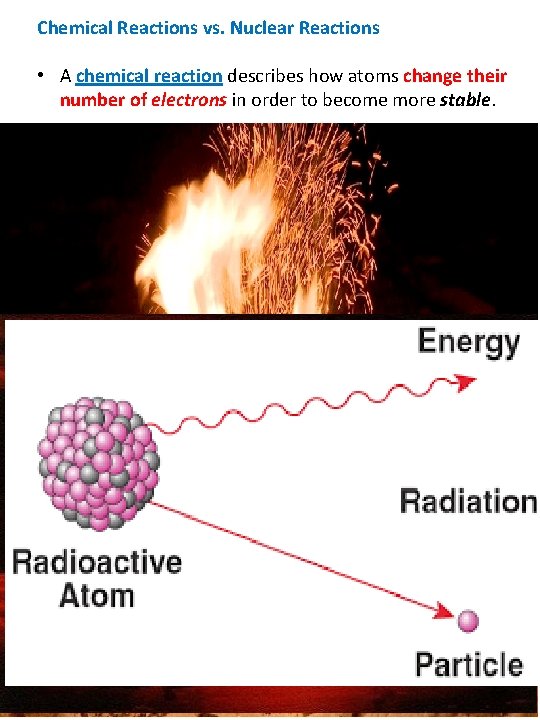
Chemical Reactions vs. Nuclear Reactions • A chemical reaction describes how atoms change their number of electrons in order to become more stable. • A nuclear reaction (or decay) describes a change in nucleus – that is a change in the number of protons & neutrons to become more stable. o The nucleus of an unstable atom releases radiation (matter and sometimes energy) during the process of nuclear decay to become stable. Types of Radioactive Emission & Symbols 1. Neutron (n) : 1 n 0 2. Alpha Particle (α): 2 protons & 2 neutrons released 4 2 He 2+ or 4 2 α 2+

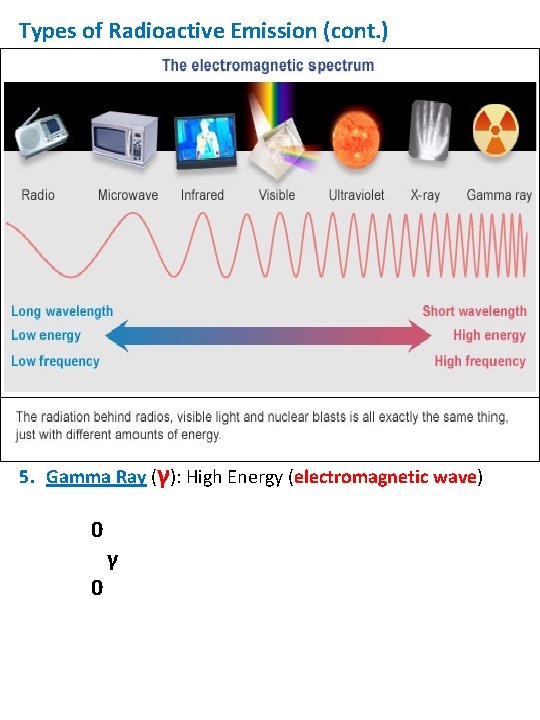
Types of Radioactive Emission (cont. ) 3. Beta Particle (β-) : Particle with mass and charge = to an Electron 0 e -1 or 0 -1 β 4. Positron: (β+) Particle with the mass of an electron, but with a positive charge 0 e +1 5. Gamma Ray (γ): High Energy (electromagnetic wave) 0 0 γ

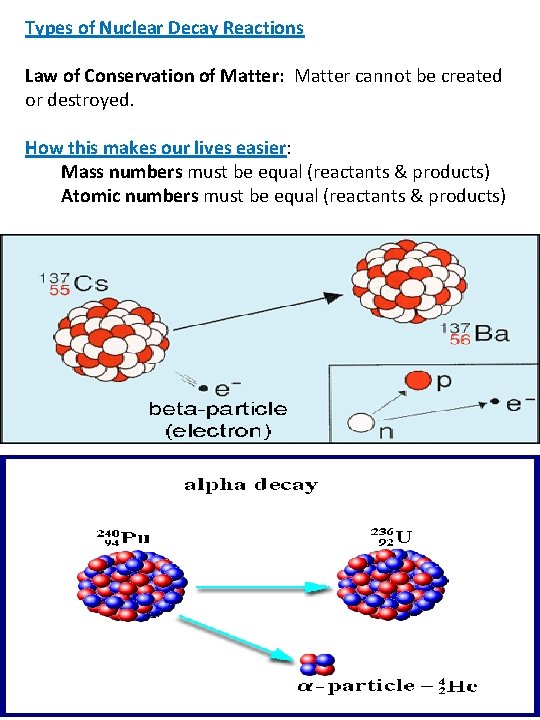
Types of Nuclear Decay Reactions Law of Conservation of Matter: Matter cannot be created or destroyed. How this makes our lives easier: Mass numbers must be equal (reactants & products) Atomic numbers must be equal (reactants & products) REACTANTS → PRODUCTS 1. Alpha Decay (α) : emission of an alpha particle Decay of Uranium- 238 by alpha decay 238 92 U → 4 2 He 2+ 234 + Th 90 2. Beta Decay(β-): emission of a beta particle Decay of Potassium-40 by beta decay 40 19 K → 0 -1 e + 40 20 Ca ** Too many neutrons in nucleus → neutron becomes proton by emitting a Beta particle (electron)

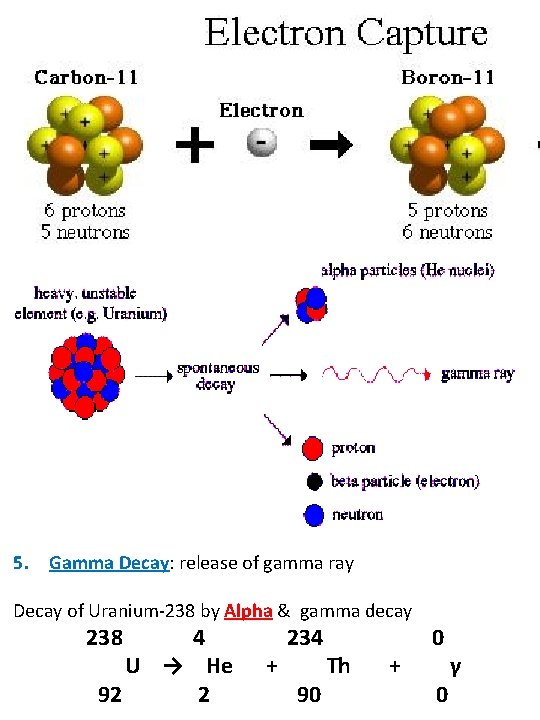
3. Positron Decay (β+): emission of a positive electron Decay of Sodium-22 by positron decay 22 11 Na → 0 +1 e + 22 10 Ne ** Too few neutrons in nucleus → proton is changed to neutron by emitting a positron (cousin to electron) 4. Electron Capture (EC): an electron is added to the nucleus Decay of Beryllium-7 by electron capture 7 Be 4 + 0 7 e -1 → Li 3 ** Too few neutrons in nucleus → captured electron joins with proton to form neutron 5. Gamma Decay: release of gamma ray Decay of Uranium-238 by Alpha & gamma decay 238 4 U → He 92 2 + 234 90 Th + 0 0 γ

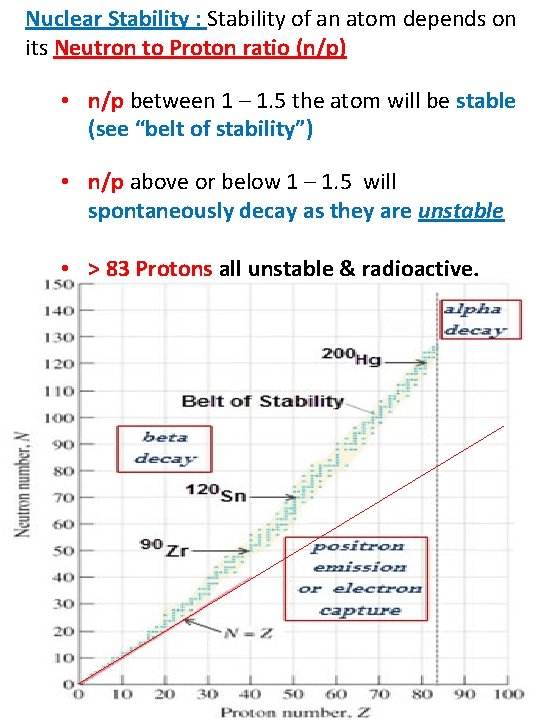
Nuclear Stability : Stability of an atom depends on its Neutron to Proton ratio (n/p) • n/p between 1 – 1. 5 the atom will be stable (see “belt of stability”) • n/p above or below 1 – 1. 5 will spontaneously decay as they are unstable • > 83 Protons all unstable & radioactive.

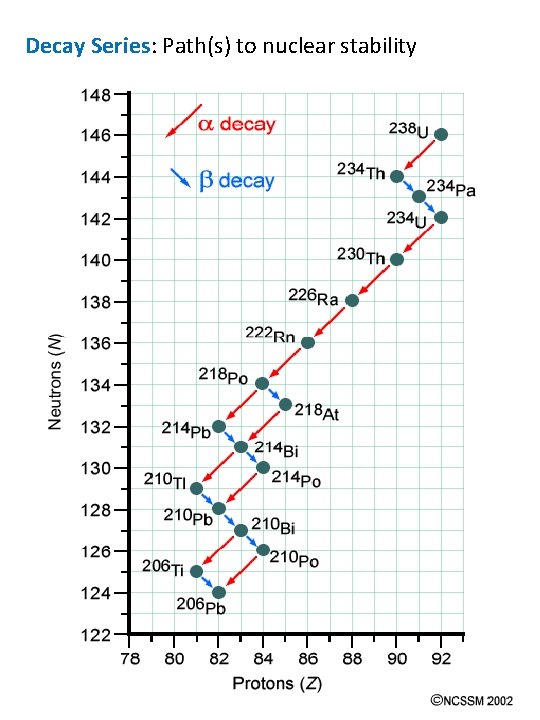
Decay Series: Path(s) to nuclear stability

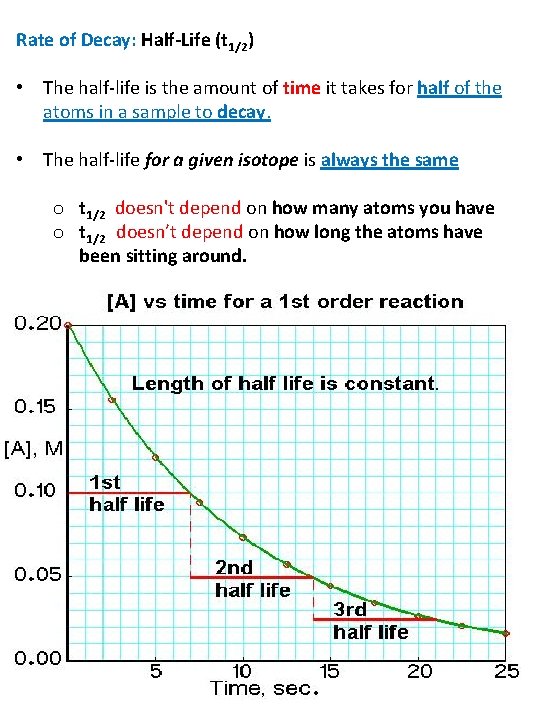
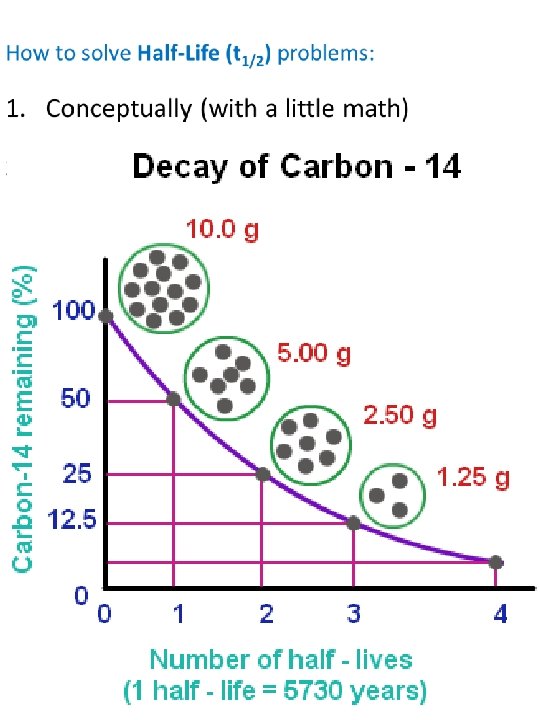
Rate of Decay: Half-Life (t 1/2) • The half-life is the amount of time it takes for half of the atoms in a sample to decay. • The half-life for a given isotope is always the same o t 1/2 doesn't depend on how many atoms you have o t 1/2 doesn’t depend on how long the atoms have been sitting around.


Nuclear Change or “Transmutation”: converting atom of 1 element to another. Two methods: 1. Nuclear decay reaction: α-decay, β-decay…. 2. Bombard the nucleus of an atom with a particle Method #2 Fission & Fusion Fission: Bombard (hit) the nucleus of a heavy isotope with a neutron causing the nucleus to split producing 2 lighter isotopes, 2 -3 more neutrons, and Energy 235 1 92 141 1 U + n → Kr + Ba + 3 n + Energy 92 0 36 56 0 Fusion: Collide two light isotopes together causing them to fuse together producing a heaver isotope and Energy 2 H 1 + (deuterium) 3 H → 1 (tritium) 4 He + Energy 2 ** After fusion, some of the mass is converted into energy. E = mc 2