Chemical Reactions The Rearranging of Atoms Rearranging Atoms



- Slides: 19

Chemical Reactions: The Rearranging of Atoms

Rearranging Atoms When two or more atoms combine and bond to form a new substance, a chemical change occurs.

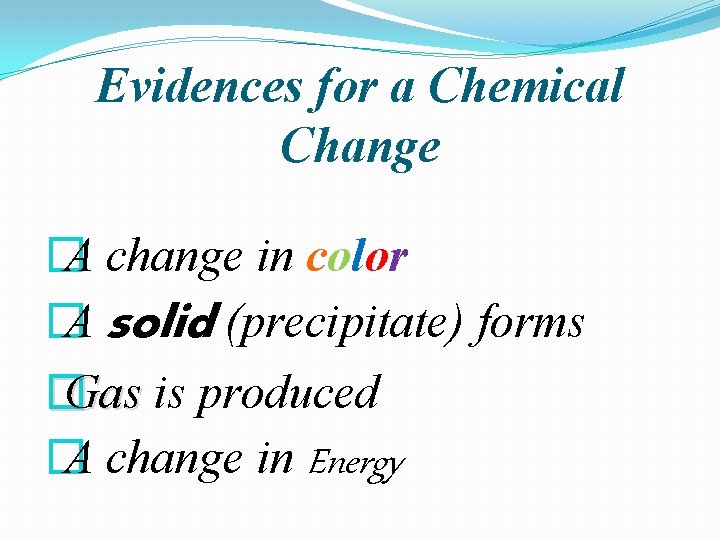
Evidences for a Chemical Change � A change in color � A solid (precipitate) forms � Gas is produced � A change in Energy

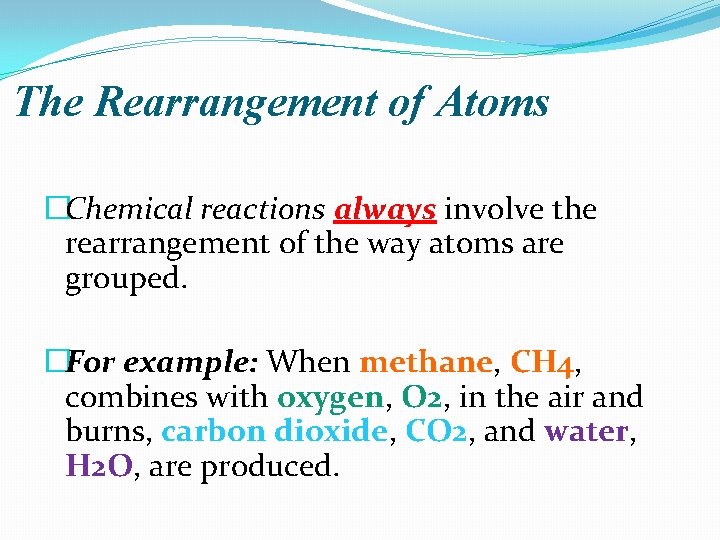
The Rearrangement of Atoms �Chemical reactions always involve the rearrangement of the way atoms are grouped. �For example: When methane, CH 4, combines with oxygen, O 2, in the air and burns, carbon dioxide, CO 2, and water, H 2 O, are produced.

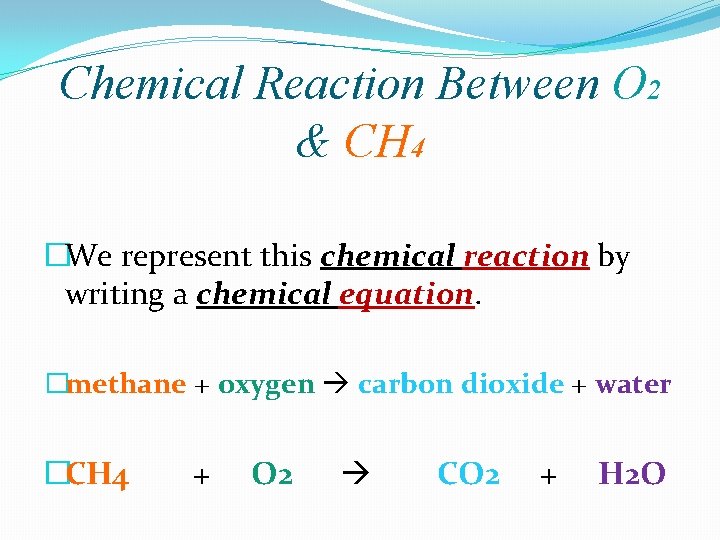
Chemical Reaction Between O 2 & CH 4 �We represent this chemical reaction by writing a chemical equation. �methane + oxygen carbon dioxide + water �CH 4 + O 2 CO 2 + H 2 O

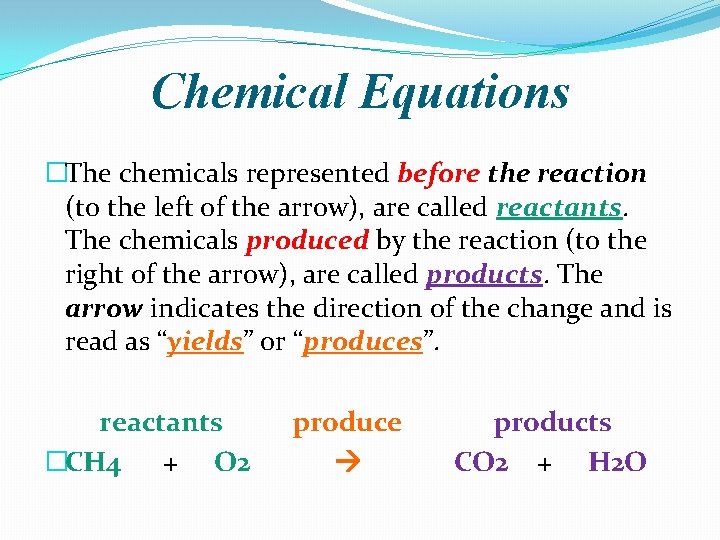
Chemical Equations �The chemicals represented before the reaction (to the left of the arrow), are called reactants. The chemicals produced by the reaction (to the right of the arrow), are called products. The arrow indicates the direction of the change and is read as “yields” or “produces”. reactants �CH 4 + O 2 produce products CO 2 + H 2 O

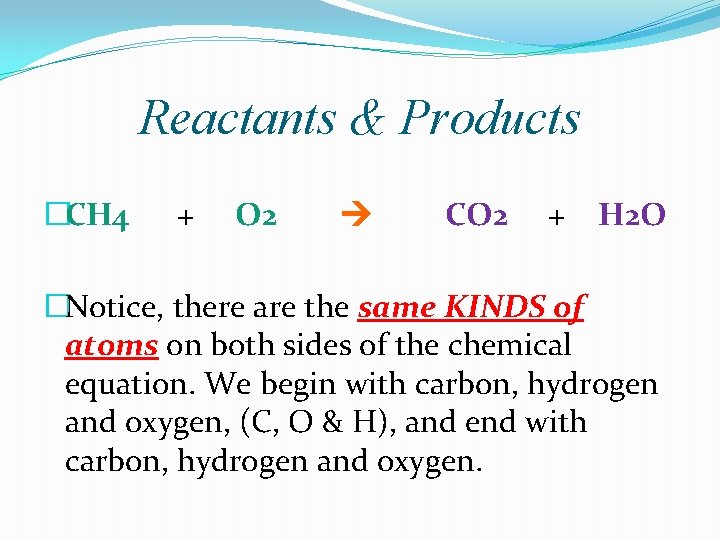
Reactants & Products �CH 4 + O 2 CO 2 + H 2 O �Notice, there are the same KINDS of atoms on both sides of the chemical equation. We begin with carbon, hydrogen and oxygen, (C, O & H), and end with carbon, hydrogen and oxygen.

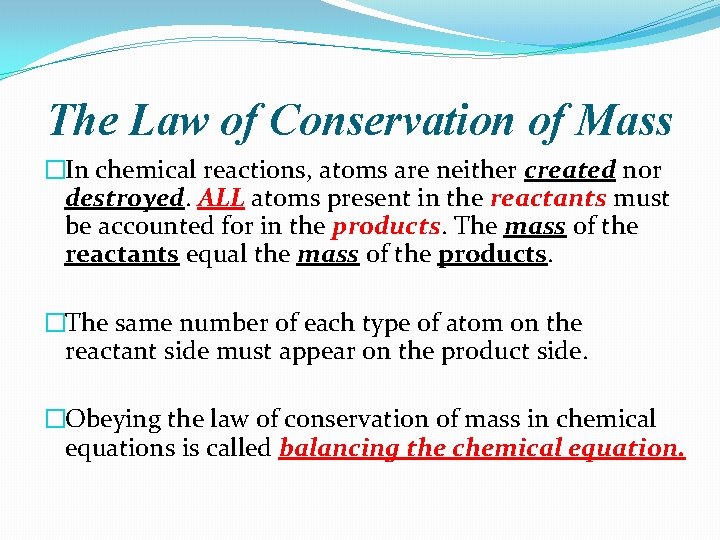
The Law of Conservation of Mass �In chemical reactions, atoms are neither created nor destroyed. ALL atoms present in the reactants must be accounted for in the products. The mass of the reactants equal the mass of the products. �The same number of each type of atom on the reactant side must appear on the product side. �Obeying the law of conservation of mass in chemical equations is called balancing the chemical equation.

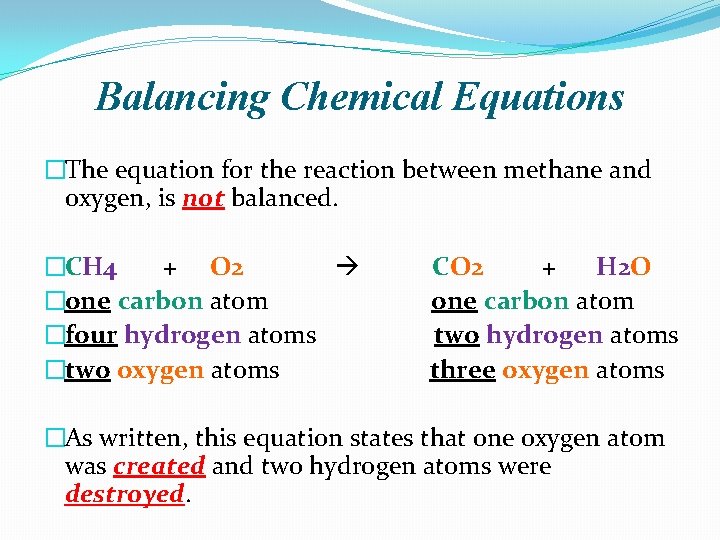
Balancing Chemical Equations �The equation for the reaction between methane and oxygen, is not balanced. �CH 4 + O 2 �one carbon atom �four hydrogen atoms �two oxygen atoms CO 2 + H 2 O one carbon atom two hydrogen atoms three oxygen atoms �As written, this equation states that one oxygen atom was created and two hydrogen atoms were destroyed.

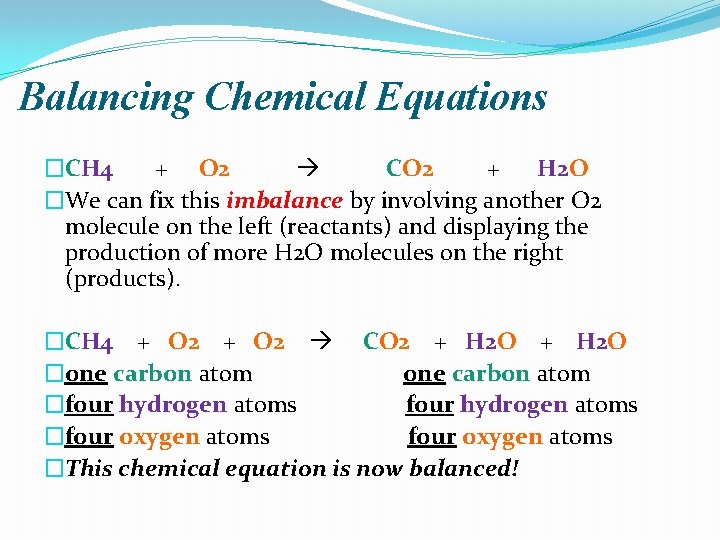
Balancing Chemical Equations �CH 4 + O 2 CO 2 + H 2 O �We can fix this imbalance by involving another O 2 molecule on the left (reactants) and displaying the production of more H 2 O molecules on the right (products). �CH 4 + O 2 CO 2 + H 2 O �one carbon atom �four hydrogen atoms �four oxygen atoms �This chemical equation is now balanced!

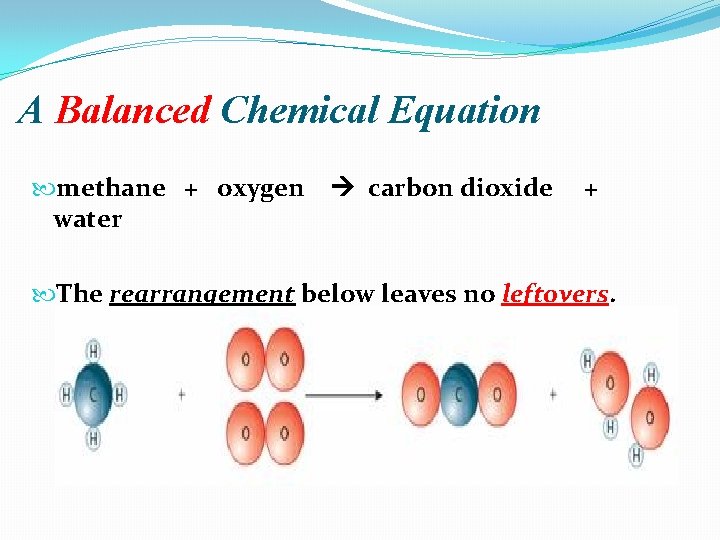
A Balanced Chemical Equation methane + oxygen carbon dioxide water + The rearrangement below leaves no leftovers.

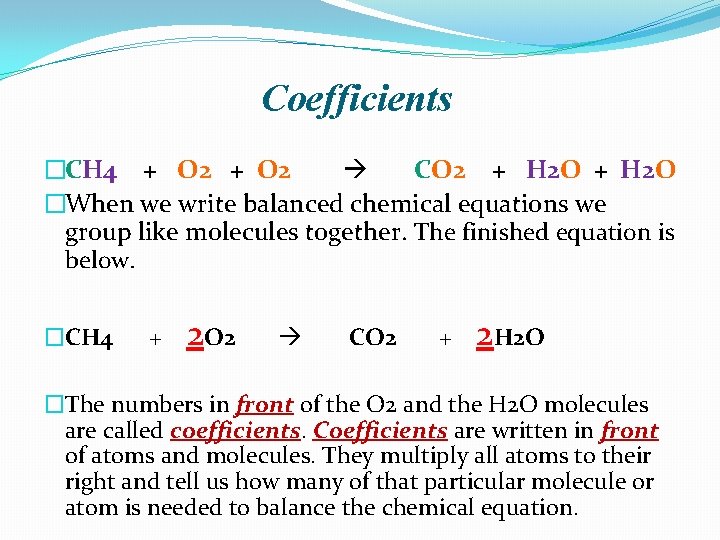
Coefficients �CH 4 + O 2 CO 2 + H 2 O �When we write balanced chemical equations we group like molecules together. The finished equation is below. �CH 4 + 2 O 2 CO 2 + 2 H 2 O �The numbers in front of the O 2 and the H 2 O molecules are called coefficients. Coefficients are written in front of atoms and molecules. They multiply all atoms to their right and tell us how many of that particular molecule or atom is needed to balance the chemical equation.

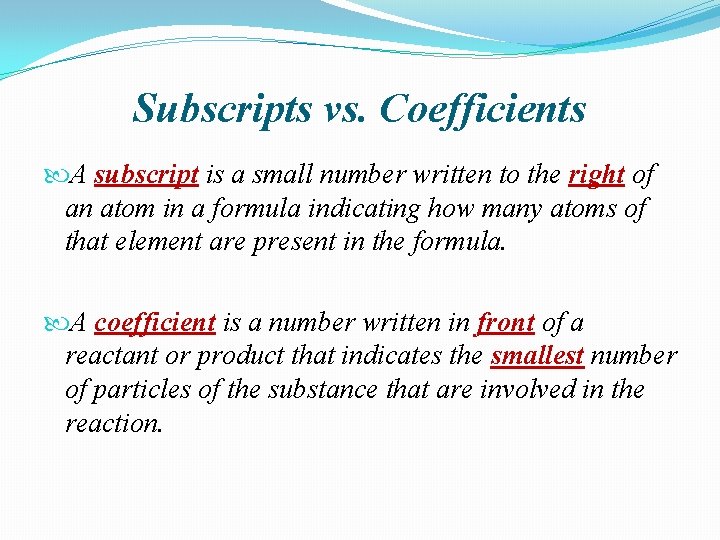
Subscripts vs. Coefficients A subscript is a small number written to the right of an atom in a formula indicating how many atoms of that element are present in the formula. A coefficient is a number written in front of a reactant or product that indicates the smallest number of particles of the substance that are involved in the reaction.

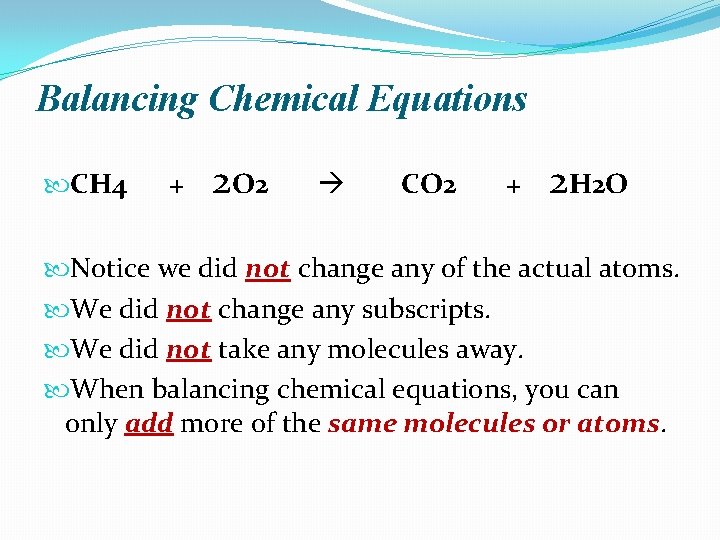
Balancing Chemical Equations CH 4 + 2 O 2 CO 2 + 2 H 2 O Notice we did not change any of the actual atoms. We did not change any subscripts. We did not take any molecules away. When balancing chemical equations, you can only add more of the same molecules or atoms.

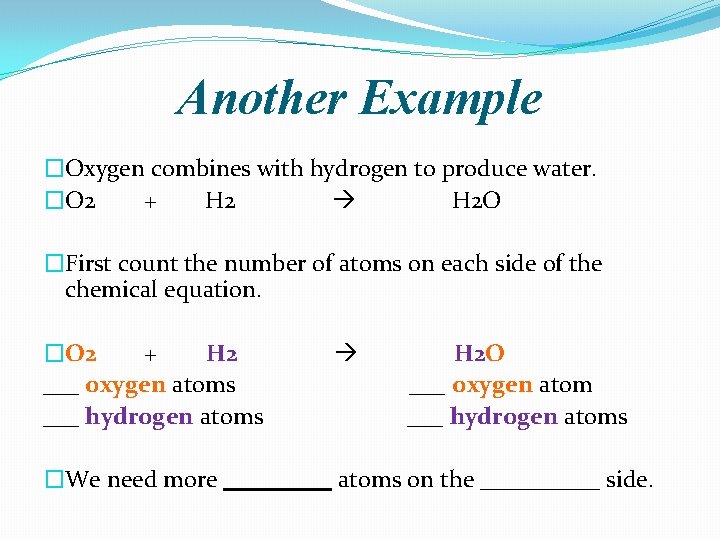
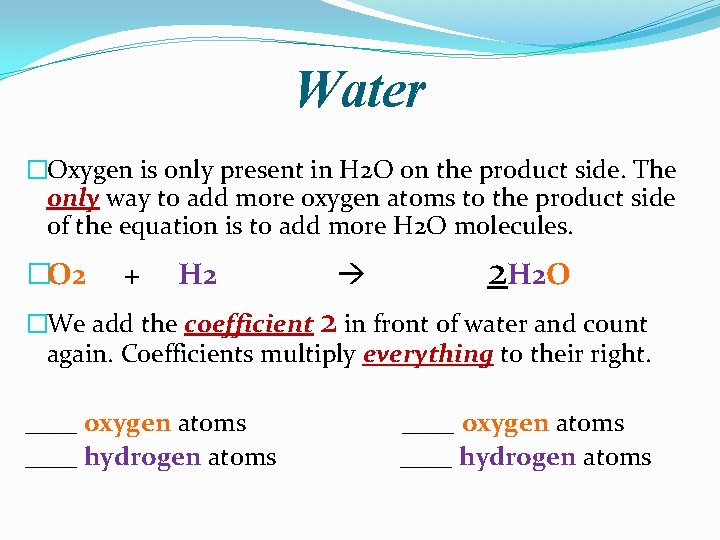
Another Example �Oxygen combines with hydrogen to produce water. �O 2 + H 2 O �First count the number of atoms on each side of the chemical equation. �O 2 + H 2 ___ oxygen atoms ___ hydrogen atoms H 2 O ___ oxygen atom ___ hydrogen atoms �We need more _____ atoms on the _____ side.

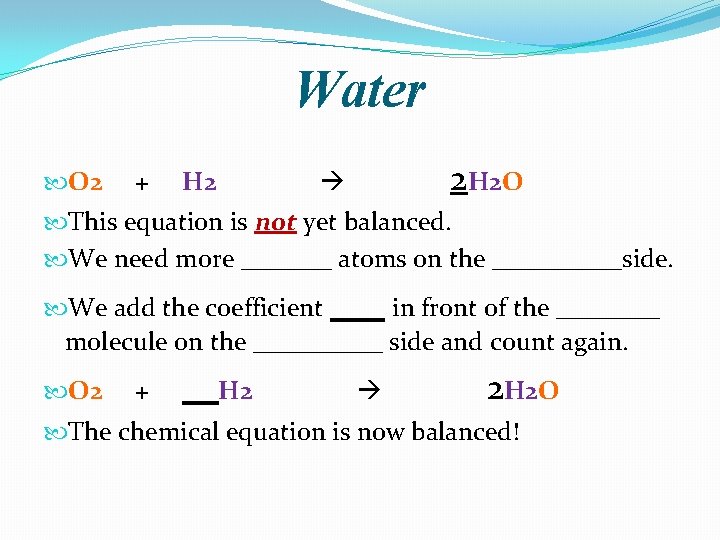
Water �Oxygen is only present in H 2 O on the product side. The only way to add more oxygen atoms to the product side of the equation is to add more H 2 O molecules. �O 2 + H 2 2 H 2 O �We add the coefficient 2 in front of water and count again. Coefficients multiply everything to their right. ____ oxygen atoms ____ hydrogen atoms

Water O 2 + H 2 2 H 2 O This equation is not yet balanced. We need more _______ atoms on the _____side. We add the coefficient ___ in front of the ____ molecule on the _____ side and count again. O 2 + __H 2 2 H 2 O The chemical equation is now balanced!

2 H 2 + O 2 2 H 2 O

Diatomics There are seven elements that are not found in nature as single atoms. The molecules formed are more stable than the individual atoms. If they are not in a chemical compound with other atoms, they pair up with another atom of their own kind. Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2 Notice how the diatomics were used in the chemical equations we looked at earlier.
 Rearranging atoms worksheet
Rearranging atoms worksheet Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Redox reactions examples
Redox reactions examples At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Homogenous solution
Homogenous solution Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Balancing chemical equations definition
Balancing chemical equations definition Reactants and products
Reactants and products Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Predicting products of chemical reactions
Predicting products of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry What is an active metal
What is an active metal Predicting products of chemical reactions
Predicting products of chemical reactions Solvent in chemical reactions
Solvent in chemical reactions