Section 2 Classifying Chemical Reactions Essential Questions What




- Slides: 9

Section 2 Classifying Chemical Reactions

Essential Questions What are the five general types of chemical reactions? How can you predict if a metal will replace another in a compound? What do the terms oxidation and reduction mean? How are redox reactions identified?

Review Vocabulary states of matter: the physical forms in which all matter exists, most commonly solid, liquid, and gas

New Vocabulary combustion reaction precipitate synthesis reaction oxidation decomposition reaction reduction single-displacement reaction double-displacement reaction

Types of Reactions You might have noticed that there all sorts of chemical reactions. In fact, there are literally millions of chemical reactions that occur every day, and scientists have described many of them and continue to describe more. Chemists have characterized these reactions and organized them into five main categories: combustion, synthesis, decomposition, single displacement (also known as single replacement), and double displacement (also known as double replacement).

Types of Reactions Shutterstock/Sinelev Burning is another word for a combustion reaction. The reactants in this reaction are carbon and hydrogen (in the wood) and oxygen (from the air). The products are carbon dioxide (CO 2) and water (H 2 O).

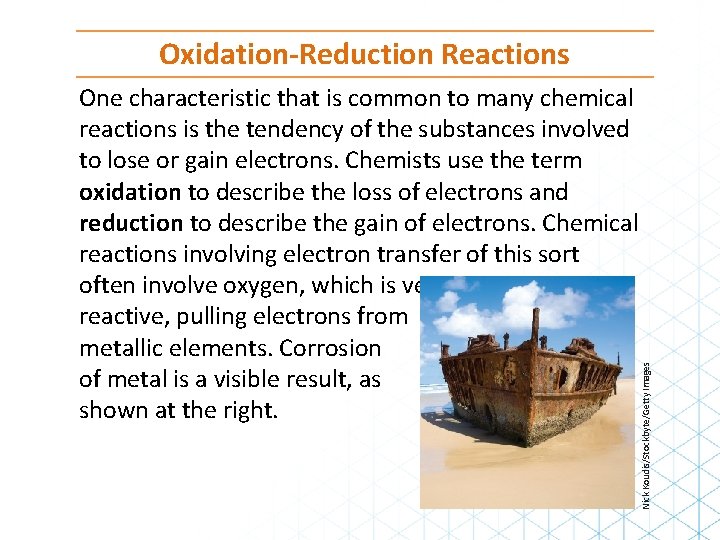
One characteristic that is common to many chemical reactions is the tendency of the substances involved to lose or gain electrons. Chemists use the term oxidation to describe the loss of electrons and reduction to describe the gain of electrons. Chemical reactions involving electron transfer of this sort often involve oxygen, which is very reactive, pulling electrons from metallic elements. Corrosion of metal is a visible result, as shown at the right. Nick Koudis/Stockbyte/Getty Images Oxidation-Reduction Reactions

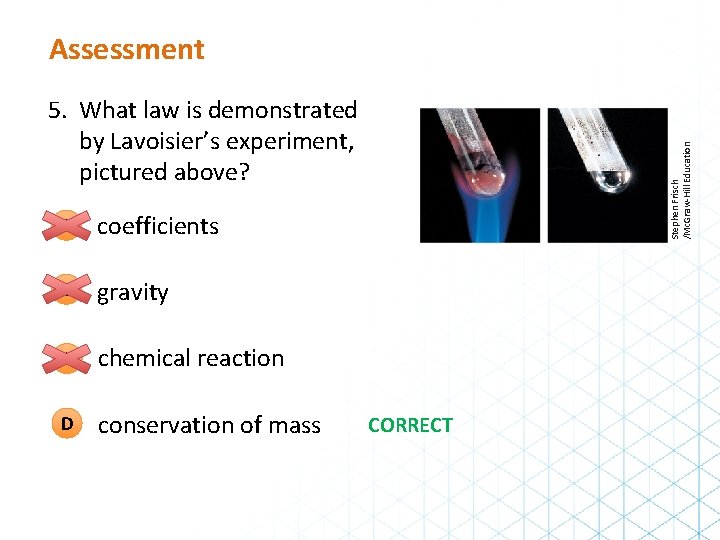
Assessment A coefficients B gravity C chemical reaction D conservation of mass Stephen Frisch /Mc. Graw-Hill Education 5. What law is demonstrated by Lavoisier’s experiment, pictured above? CORRECT

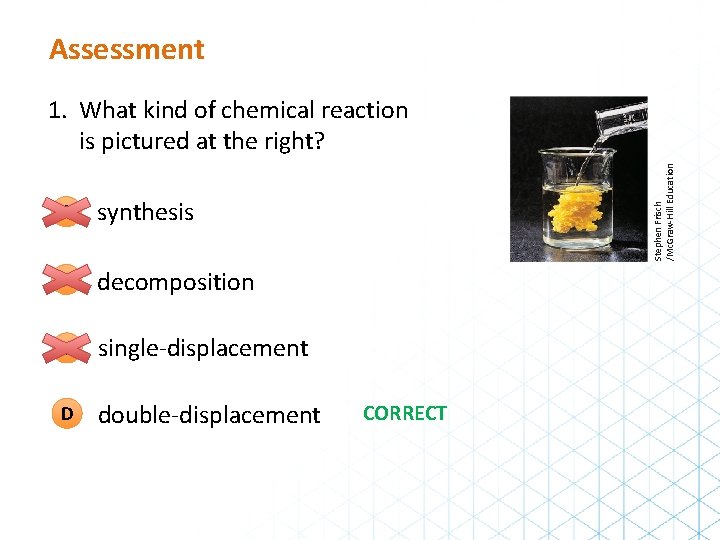
Assessment A synthesis B decomposition C single-displacement D double-displacement Stephen Frisch /Mc. Graw-Hill Education 1. What kind of chemical reaction is pictured at the right? CORRECT