Chapter 2 4 Chemical Reactions and Enzymes Chemical



- Slides: 18

Chapter 2. 4: Chemical Reactions and Enzymes

Chemical Reactions Chemical Reaction: A change of one set of chemicals into another 1. Can be slow or fast 2. Chemical reactions require collisions between molecules which makes them unstable 3. Involves changes in chemical bonds AB + CD AC + BD

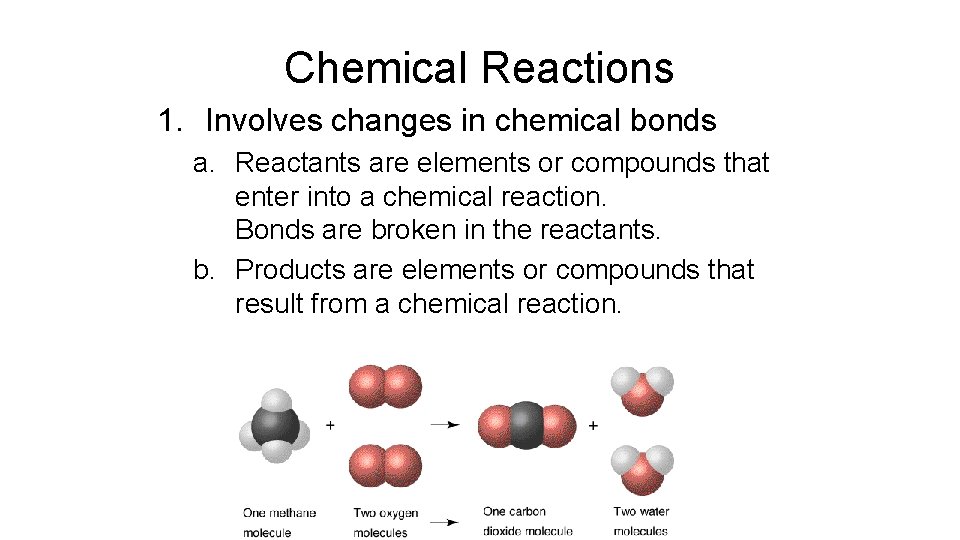
Chemical Reactions 1. Involves changes in chemical bonds a. Reactants are elements or compounds that enter into a chemical reaction. Bonds are broken in the reactants. b. Products are elements or compounds that result from a chemical reaction.

Chemical Reactions c. Whenever a reaction occurs that rearranges the atoms of molecules, bonds in the reactants must be broken and new bonds in the products must be formed.

Chemical Reactions 1. Involves changes in chemical bonds AB + CD AC + BD Which are the reactants? Which are the products? AB and CD AC and. BD

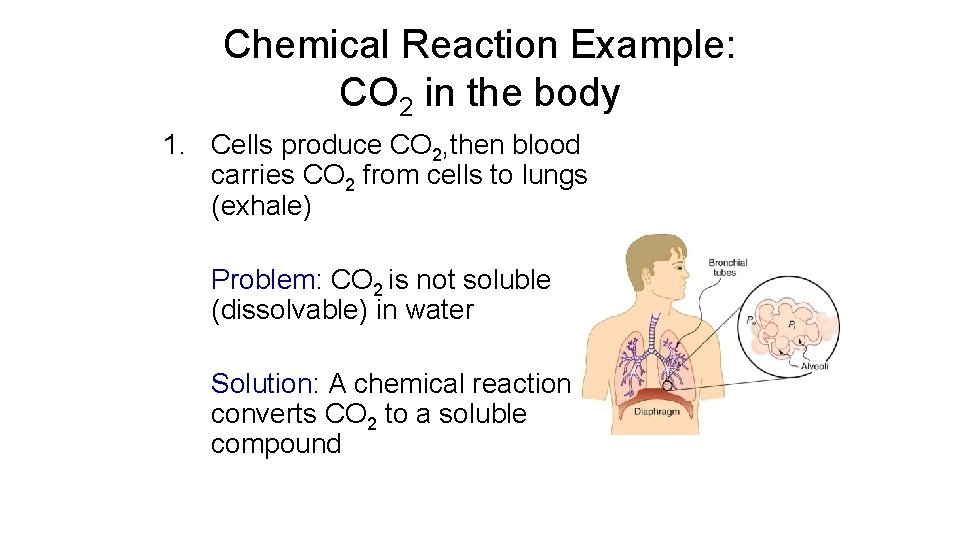
Chemical Reaction Example: CO 2 in the body 1. Cells produce CO 2, then blood carries CO 2 from cells to lungs (exhale) Problem: CO 2 is not soluble (dissolvable) in water Solution: A chemical reaction converts CO 2 to a soluble compound

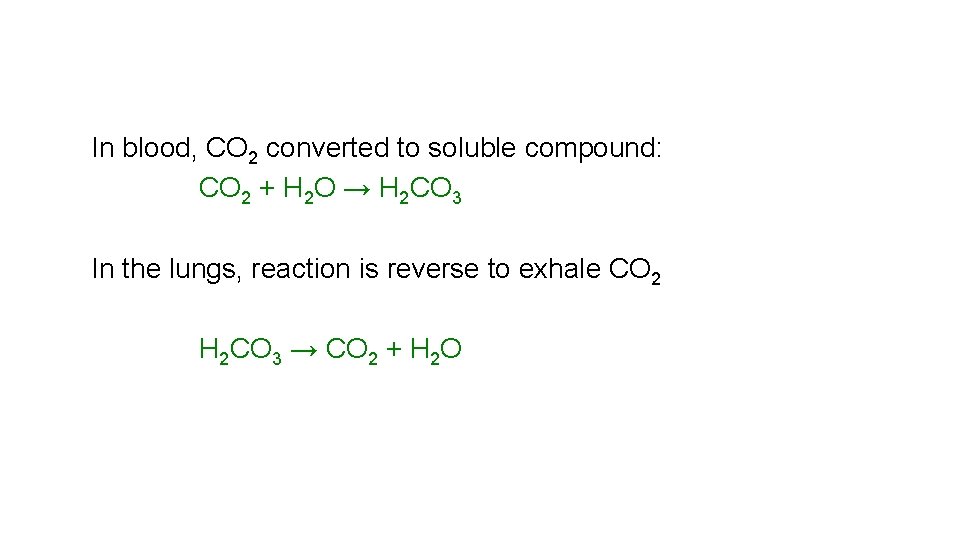
In blood, CO 2 converted to soluble compound: CO 2 + H 2 O → H 2 CO 3 In the lungs, reaction is reverse to exhale CO 2 H 2 CO 3 → CO 2 + H 2 O

Chemical reactions involve energy 1. Breaking and forming chemical bonds requires energy release or absorption 2. Reactions that release energy can occur spontaneously (but not all do) a. Energy is released as heat 3. Reactions that absorb energy will not occur without an energy source

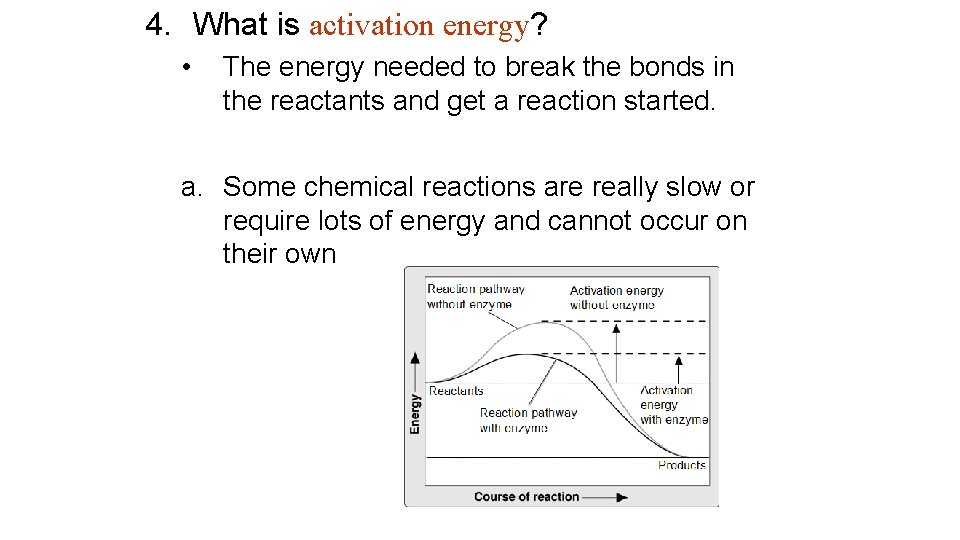
4. What is activation energy? • The energy needed to break the bonds in the reactants and get a reaction started. a. Some chemical reactions are really slow or require lots of energy and cannot occur on their own

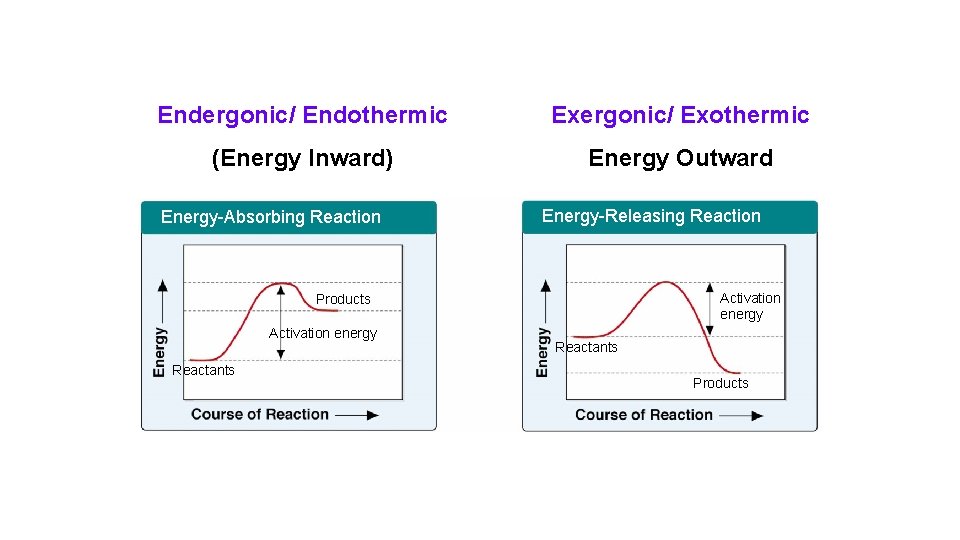
Endergonic/ Endothermic Exergonic/ Exothermic (Energy Inward) Energy Outward Energy-Absorbing Reaction Energy-Releasing Reaction Activation energy Products Activation energy Reactants Products

• Endergonic reaction – absorb free energy from the surroundings. • Exergonic reaction – have a net release of free energy. – Can occur spontaneously.

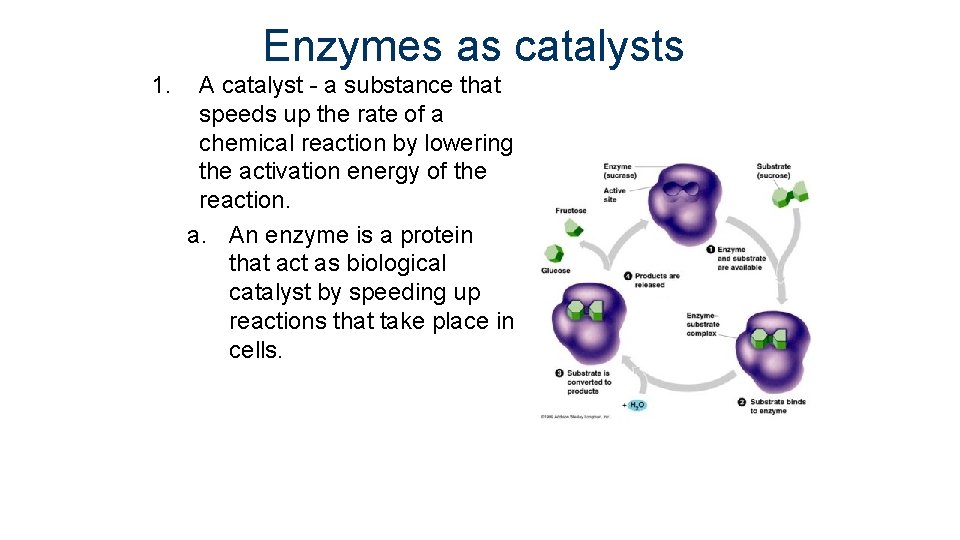
Enzymes as catalysts 1. A catalyst - a substance that speeds up the rate of a chemical reaction by lowering the activation energy of the reaction. a. An enzyme is a protein that act as biological catalyst by speeding up reactions that take place in cells.

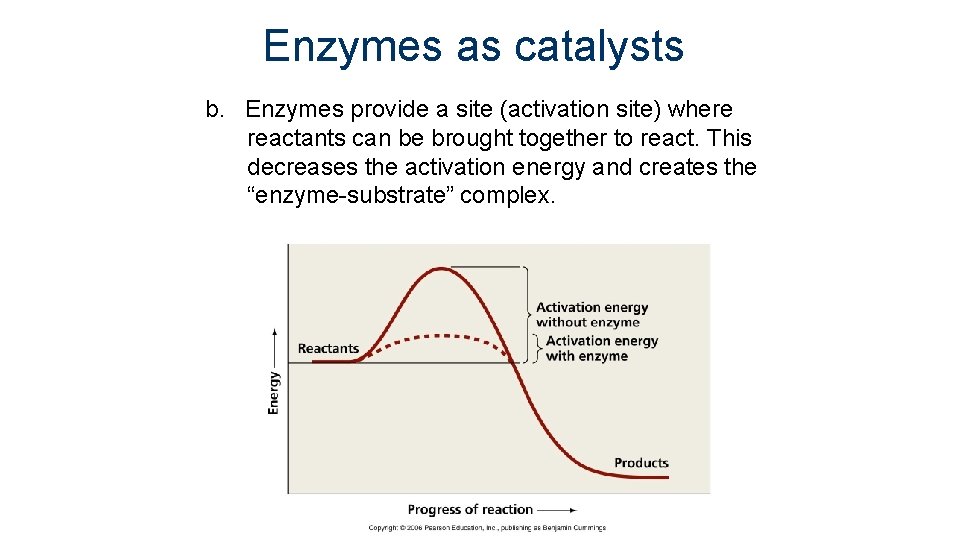
Enzymes as catalysts b. Enzymes provide a site (activation site) where reactants can be brought together to react. This decreases the activation energy and creates the “enzyme-substrate” complex.

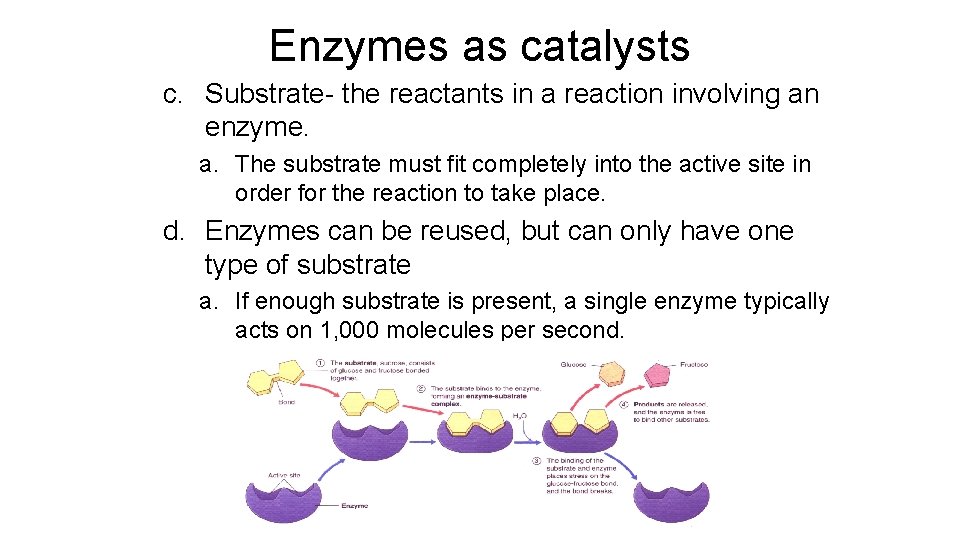
Enzymes as catalysts c. Substrate- the reactants in a reaction involving an enzyme. a. The substrate must fit completely into the active site in order for the reaction to take place. d. Enzymes can be reused, but can only have one type of substrate a. If enough substrate is present, a single enzyme typically acts on 1, 000 molecules per second.

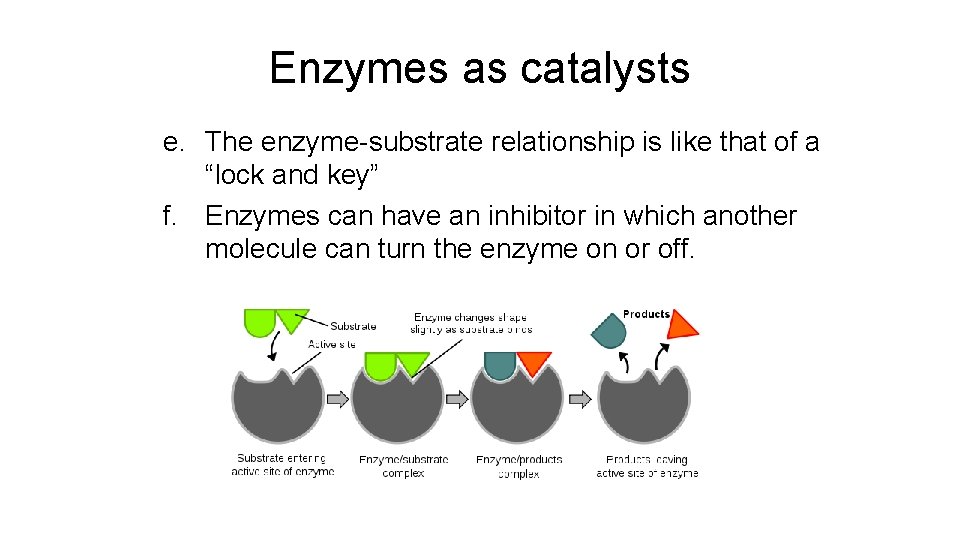
Enzymes as catalysts e. The enzyme-substrate relationship is like that of a “lock and key” f. Enzymes can have an inhibitor in which another molecule can turn the enzyme on or off.

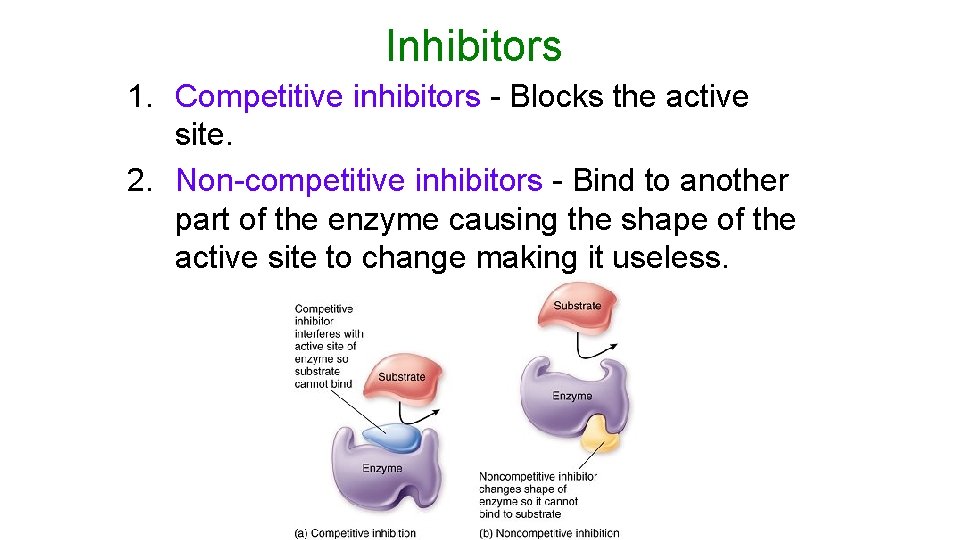
Inhibitors 1. Competitive inhibitors - Blocks the active site. 2. Non-competitive inhibitors - Bind to another part of the enzyme causing the shape of the active site to change making it useless.

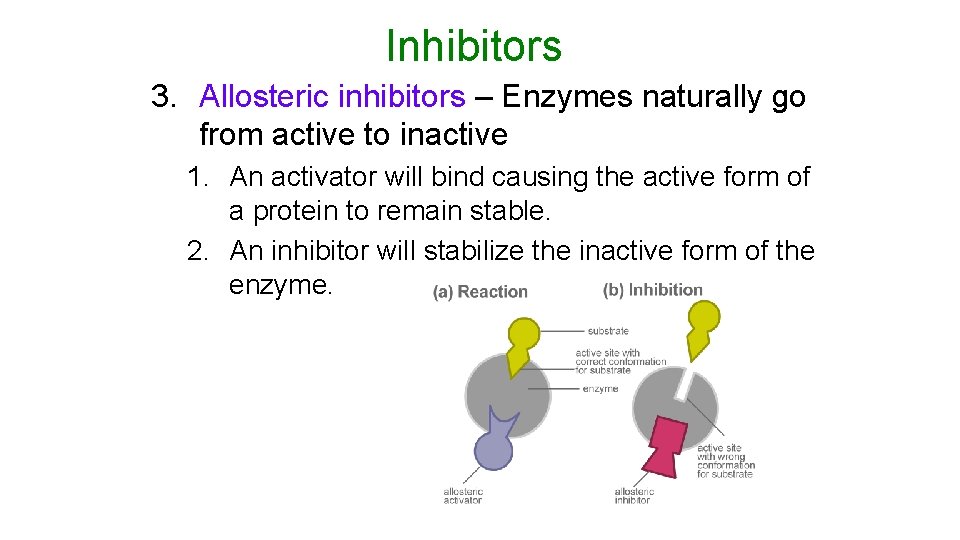
Inhibitors 3. Allosteric inhibitors – Enzymes naturally go from active to inactive 1. An activator will bind causing the active form of a protein to remain stable. 2. An inhibitor will stabilize the inactive form of the enzyme.

More on Enzymes g. Denature – When enzymes lose their shape and functionality. This can be caused by: 1. 2. 3. 4. p. H Temperature Other proteins Chemicals