Chapter 8 Solutions SOLUTION SOLVENT SOLUTE Solvent a
































- Slides: 48

Chapter 8: Solutions

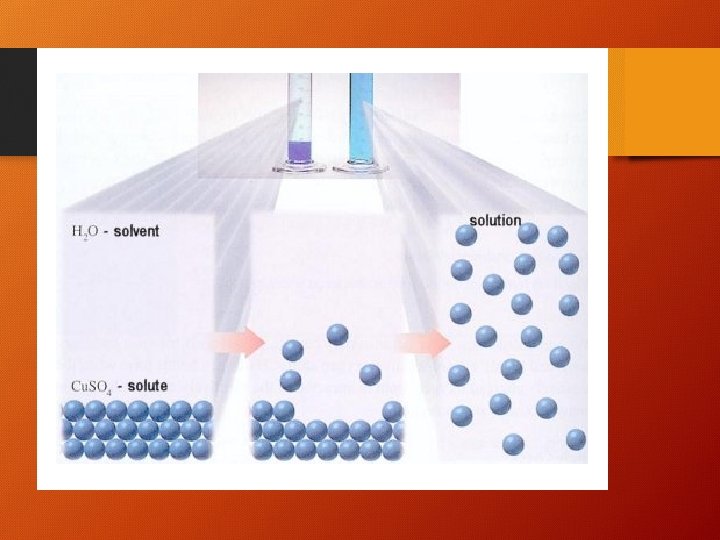
SOLUTION = SOLVENT + SOLUTE • Solvent - a substance that dissolves another substance • Or, the substance present in greater amount • Solute - a substance which is dissolved by another substance • Or, the substance present in lesser amount


Water as a solvent • Water is sometimes called the “Universal Solvent” • It is the most common solvent in nature/biological systems • Why do you think water is such a good solvent? • Polarity • Hydrogen bonding

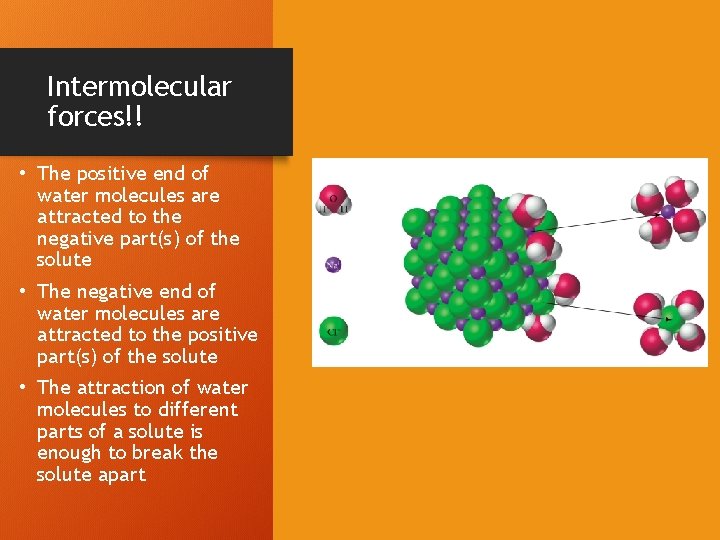
Intermolecular forces!! • The positive end of water molecules are attracted to the negative part(s) of the solute • The negative end of water molecules are attracted to the positive part(s) of the solute • The attraction of water molecules to different parts of a solute is enough to break the solute apart

Solvation • As each ion in the solute is drawn into solution, it is surrounded by water molecules • This process is called “solvation” • Solvation lessens the attraction of the solute ions to each other

Salt dissolving video • https: //www. youtube. com/watch? v=xdedxfhc p. Wo

Like dissolves like • For a solution to form, the solvent and solute molecules must be attracted to each other • “Like Dissolves Like” • Polar solvents dissolve polar solutes • Nonpolar substances are attracted to other nonpolar substances, therefore nonpolar solvents will dissolve nonpolar solutes • Examples: I 2, Hexane, Cooking Oils


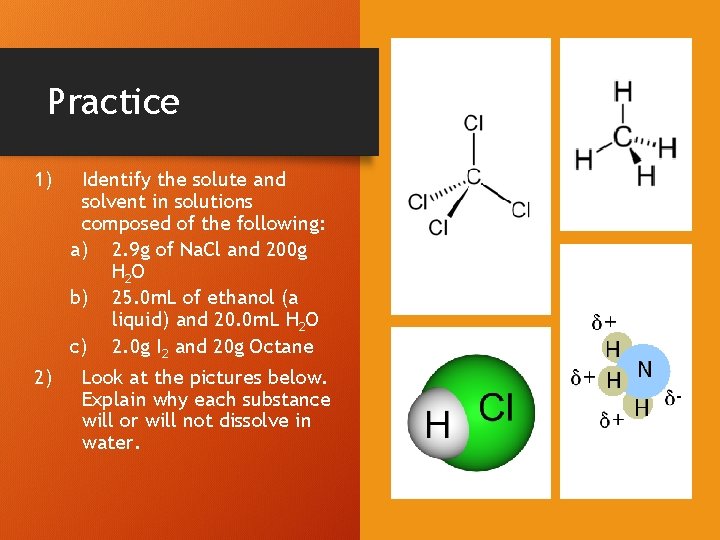
Practice 1) Identify the solute and solvent in solutions composed of the following: a) 2. 9 g of Na. Cl and 200 g H 2 O b) 25. 0 m. L of ethanol (a liquid) and 20. 0 m. L H 2 O c) 2. 0 g I 2 and 20 g Octane 2) Look at the pictures below. Explain why each substance will or will not dissolve in water.

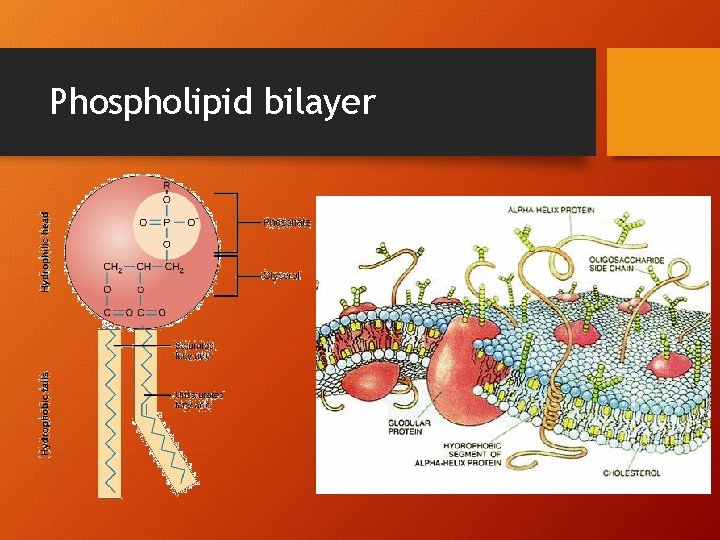
Solubility • Solubility - the amount of solute that will dissolve in a specific solvent under given conditions • Polar solutes will be more soluble in polar solvents • Non-polar solutes will be more soluble in non-polar solvents • Amphiphilic solutes will be soluble in both polar and nonpolar solvents • Have hydrophobic and hydrophilic regions • Example: Phospholipids

Phospholipid bilayer

More on solubility • Solubility is expressed in grams of solute/100 g solvent • Example: The solubility of Ethanol is 10 g/100 g H 2 O @ 23ºC • Unsaturated Solution - solution in which the solvent can dissolve more solute • Saturated Solution - solution in which the solvent cannot dissolve any more solute

• When a solution is saturated, the rate of dissolution is the same as the rate of recrystallization or precipitation

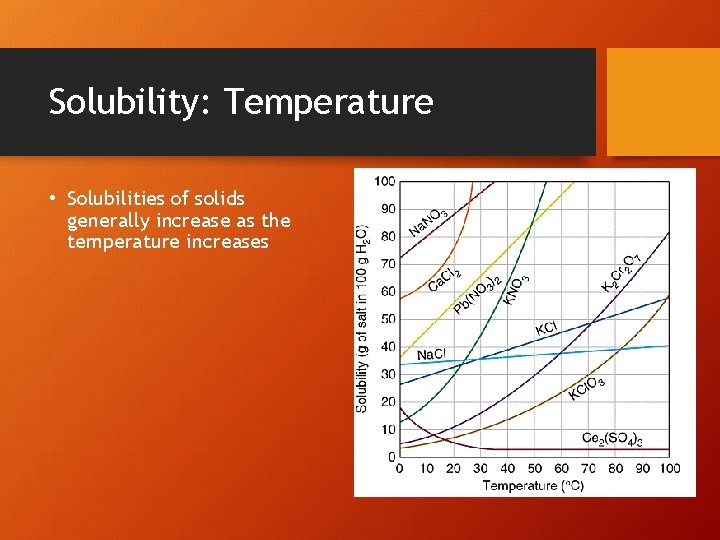
Solubility: Temperature • Solubilities of solids generally increase as the temperature increases

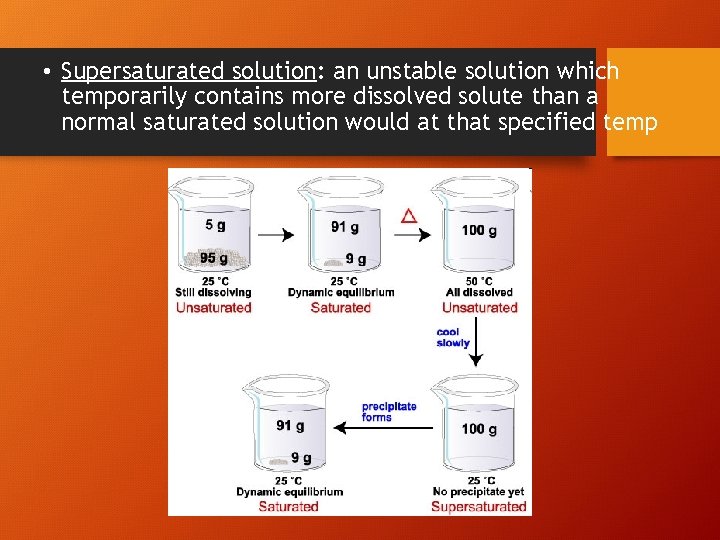
• Supersaturated solution: an unstable solution which temporarily contains more dissolved solute than a normal saturated solution would at that specified temp

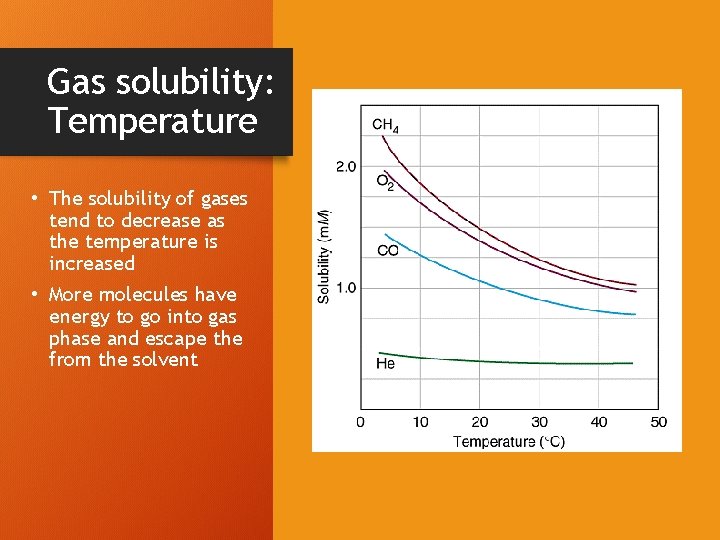
Gas solubility: Temperature • The solubility of gases tend to decrease as the temperature is increased • More molecules have energy to go into gas phase and escape the from the solvent

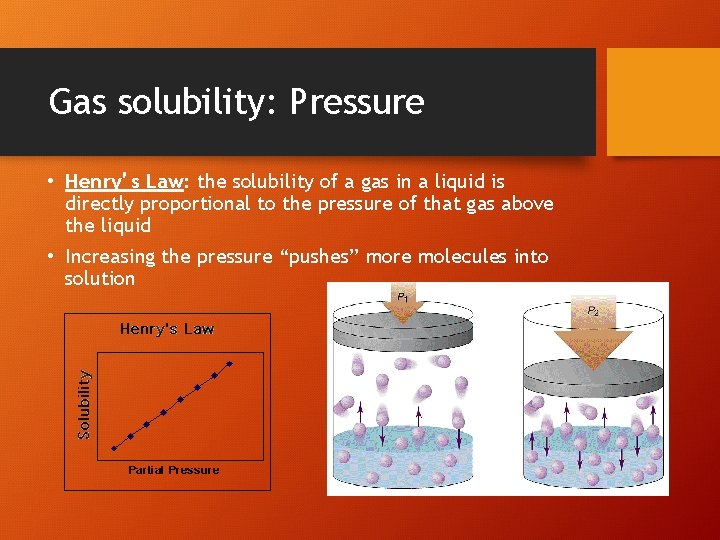
Gas solubility: Pressure • Henry’s Law: the solubility of a gas in a liquid is directly proportional to the pressure of that gas above the liquid • Increasing the pressure “pushes” more molecules into solution

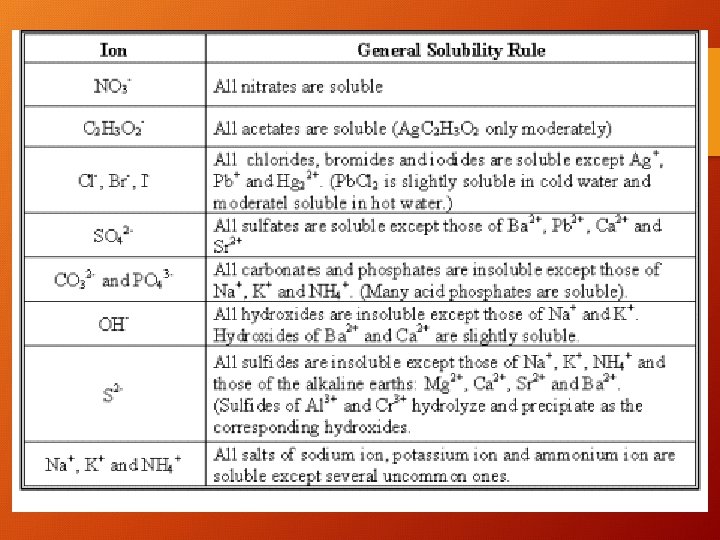
Soluble and Insoluble Salts • How do you know that Na. Cl is soluble in water? • What about KBr? Is it soluble? • What about Ag. Cl? • You can tell if a salt will be soluble or insoluble in water by looking up the cation and the anion on a solubility chart


Practice Which of the following salts is soluble in water? Which is insoluble? 1) 2) 3) 4) 5) 6) Ag. Cl KNO 3 Sr. CO 3 Ca. SO 4 Li. OH Na. OH

Concentrations • Concentration - amount of solute per amount of solution • There are various ways to express concentration • Dilute solution – small amount of solute relative to the amount of solvent • Concentrated solution– large amount of solute relative to the amount of solvent

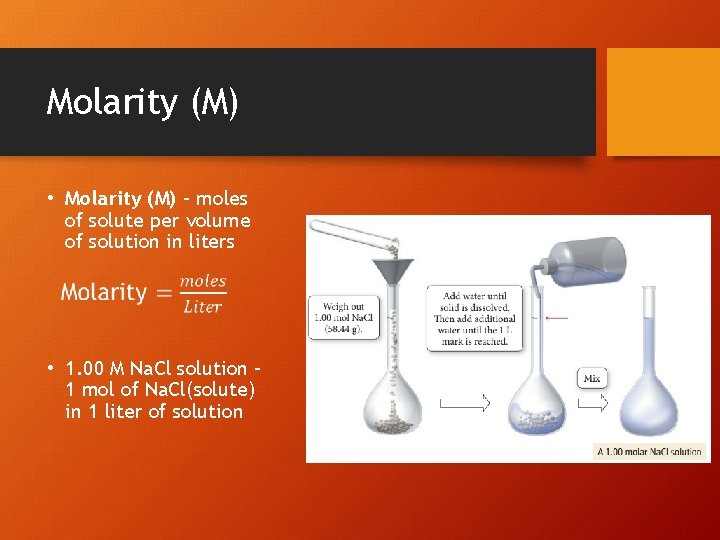
Molarity (M) • Molarity (M) – moles of solute per volume of solution in liters • 1. 00 M Na. Cl solution – 1 mol of Na. Cl(solute) in 1 liter of solution

Practice • If you need to make 2. 50 liters of 1. 65 M Na. Cl solution, how many moles of Na. Cl do you need?

Mass/volume percent (%m/v) • Mass/volume percent (% m/v) or (w/v %) mass of the solute divided by the volume of solution and multiplied by 100% • Units of mass are grams • Units of volume are m. L

Practice • You dissolve 5. 66 g KBr in enough H 2 O to make 27 m. L of solution. What is the mass/volume percent for the solution?

Percent by mass (%m/m) • Percent by Mass (%m/m or %w/w) mass of solute in mass of solution multiplied by 100% • Mass % = mass solute/mass solution x 100 • Remember: mass solution=mass solute + mass solvent

Practice • You have a 10. 0 % (m/m)% sucrose solution. If you used 25. 0 g sucrose to make the solution, how much solvent (water) did you use in grams?

Percent by volume (% v/v) • Percent by volume (% v/v): volume of solute divided by volume of solution multiplied by 100 • VP = volume solute/volume solution x 100 • Units of volume must be the same for both solute and solvent

Practice • A 750 m. L bottle of vodka has an alcohol concentration of 40% (v/v). How much volume of alcohol is present in a bottle?

Parts per million (ppm) • Parts per million (ppm) – useful for expressing extremely low concentrations • Mass or volume can be used to solve for ppm

Practice • Seabirds feed on fish contaminated with the pesticide DDT accumulate an average of 25 ppm of DDT in their fatty tissues. If an average osprey weighs 1. 36 kg, how many grams of DDT are present in its body

Dilutions • Dilution - the process by which more solvent is added to a solution in order to lower the concentration • Adding more volume of solvent makes a solution less concentrated • C 1 V 1 = C 2 V 2 • C = Concentration • V = Volume

Problems 1) Joan has 50 m. L of a 0. 498 M glucose solution. She’d like to dilute this to a 0. 250 M glucose solution. What will her final volume be? 2) A nurse wants to prepare a 1. 0% (m/v) silver nitrate solution from 24 m. L or a 3. 0% stock solution of silver nitrate. How much water should be added to the 24 m. L solution?

Mixtures: Homogeneous • Homogeneous Mixture: a mixture in which the composition is the same throughout • Only one phase present • Cannot see interface • Particle size less than 1 nm • Often called “solutions”

Mixtures: Heterogeneous • Heterogeneous Mixture - a mixture in which the particles of each component remain separate and can be observed as individual substances • See distinct phases • See interface • Solute particle size above 200 nm

Mixtures: Suspensions • Heterogeneous mixtures • Contain particles large enough to be trapped by filters and semipermeable membranes, but small enough to stay suspended for a while before settling out. • Examples: Muddy water, liquid medications, paint • Most things that direct you to shake or stir before using

Mixtures: Colloids • Mixtures with properties intermediate between heterogeneous and homogeneous mixtures • Particles tend to be small enough to pass through filters, but too large to pass through semipermeable membranes • Solute size (1 nm-200 nm) • Examples: Jell-O, Fog, Dust in air, Mayo

The Tyndall Effect • Named after the Irish scientist John Tyndall • Describes the light scattering effect cause by particles in a colloid • Can see the beam of light • Can see the particles in the colloid • Used to distinguish colloids from other types of mixtures

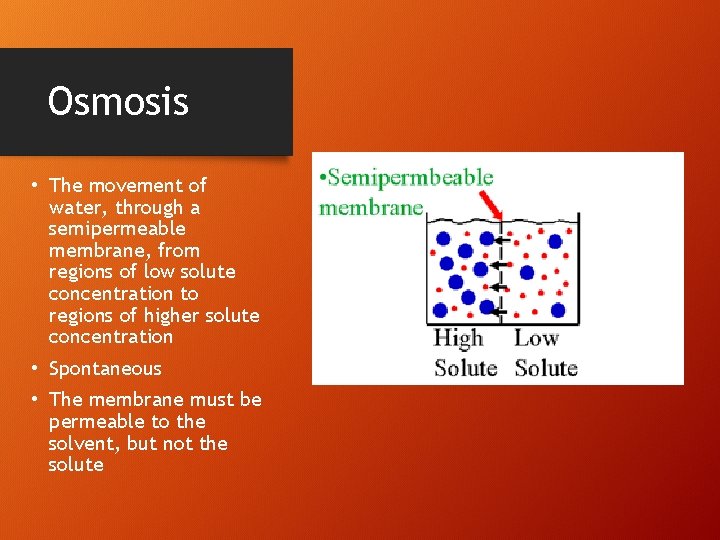
Osmosis • The movement of water, through a semipermeable membrane, from regions of low solute concentration to regions of higher solute concentration • Spontaneous • The membrane must be permeable to the solvent, but not the solute

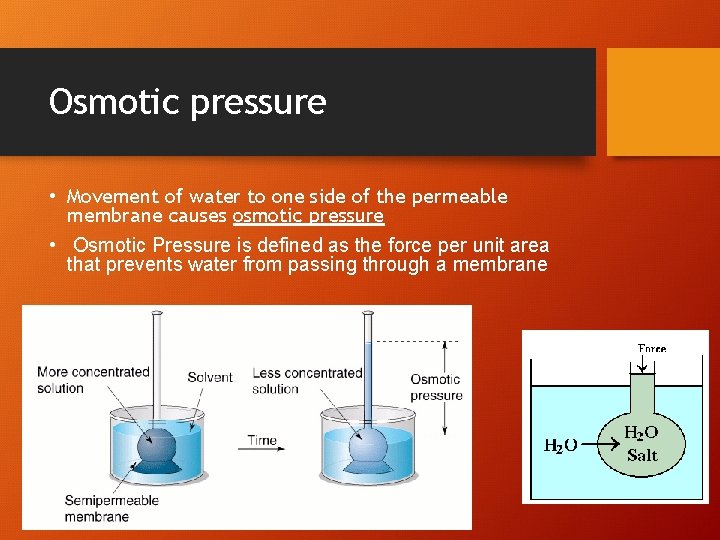
Osmotic pressure • Movement of water to one side of the permeable membrane causes osmotic pressure • Osmotic Pressure is defined as the force per unit area that prevents water from passing through a membrane

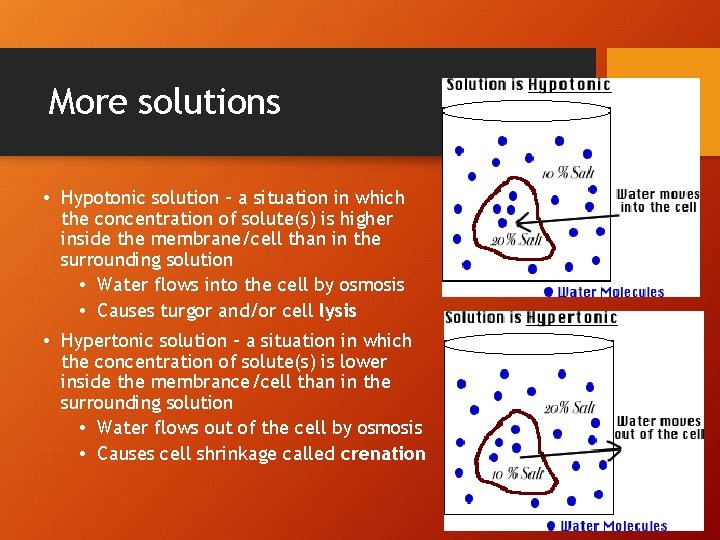
More solutions • Hypotonic solution - a situation in which the concentration of solute(s) is higher inside the membrane/cell than in the surrounding solution • Water flows into the cell by osmosis • Causes turgor and/or cell lysis • Hypertonic solution - a situation in which the concentration of solute(s) is lower inside the membrance/cell than in the surrounding solution • Water flows out of the cell by osmosis • Causes cell shrinkage called crenation

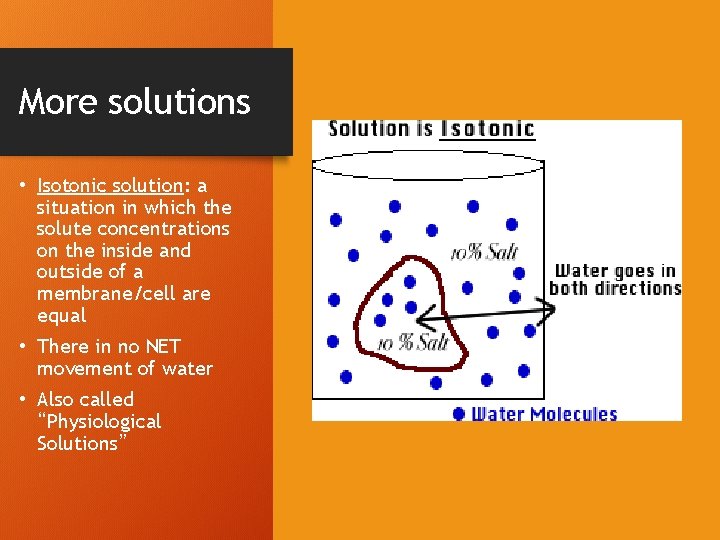
More solutions • Isotonic solution: a situation in which the solute concentrations on the inside and outside of a membrane/cell are equal • There in no NET movement of water • Also called “Physiological Solutions”

Colligative properties: Boiling Point elevation • Properties that depend on the concentration of the solute, but not its identity • A more concentrated solution will increase the boiling point of the solvent • One mole of solute particles raises the boiling point of one kilogram of water by 0. 51°C • 1 mol of H 2 O = 1 mole of solute particles • 1 mol of Na. Cl = 2 moles of solute particles • In solution, Na. Cl dissociates into Na+ and Cl- particles

Colligative properties: Freezing point depression • More concentrated solutions will lower the freezing point of the solvent • One mole of solute particles decreases the freezing point of one kilogram of water by 1. 86°C

Problems • If you add 10 moles of pentane (C 5 H 12) how much will the boiling point of water increase by? • If you add 5 moles of Na. Cl to 1 kg of water, what will the new boiling point be?

Chapter 8 checklist • Know how to determine what a solute or a solvent is in a solution • Know how to identify polar vs non polar compounds in relations to the “like dissolves like” rule • Know how temperature affects solubility of liquids and gases differently • Know henry’s law, how pressure affects gas solubility • Know solution types (saturated, super satured, unsaturated) • General Solubility rules for salts • Know how to use each concentration unit to solve for the components/properties of a solution • Concentrations- mol/L, m/m%, m/v%, v/v%

Chapter 8 checklist continued • Know how to do dilution calculations • Know the differences between mixtures, colloids, and suspensions • Know what osmosis is and what osmotic pressure is • Know how to raise the boiling point or lower the freezing point of a solution and understand the reasoning behind why it does that