Matter and Change ACT Opener Keep the opener



















- Slides: 26

Matter and Change

ACT Opener Keep the opener sheet with you for two weeks Write the question and answer it Which statement is always true for a compound? A. It is homogeneous B. It contains only 1 element C. Its chemical composition varies D. It decomposes by physical means

Review Growth Mindset Keep trying until you get it Then keep making it better Test your mindset Which mindset are you? Which mindset do you want?

Unit 1: Matter and Change “I Can” statements Standards That for the entire unit is what you will be tested on Keep in your notebook

Unit Pre-Test Answer Do the Pre-Test questions your best Similar to Unit test

Opener Make sure to copy question and answers What is the name given to anything that takes up space and has mass A. Compound B. Matter C. Mixture D. Substance

Review Standards Pre-Test and “I can’s”

Notes (keep in binder) Chemistry- the study of the composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany these processes Broken into 6 types

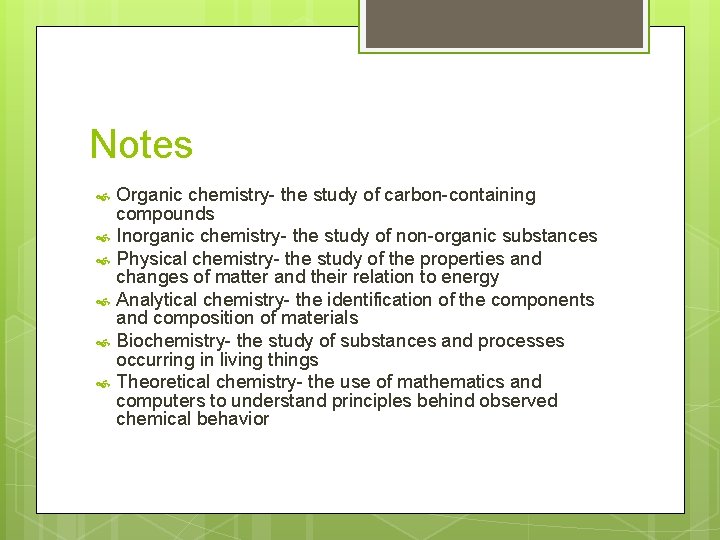
Notes Organic chemistry- the study of carbon-containing compounds Inorganic chemistry- the study of non-organic substances Physical chemistry- the study of the properties and changes of matter and their relation to energy Analytical chemistry- the identification of the components and composition of materials Biochemistry- the study of substances and processes occurring in living things Theoretical chemistry- the use of mathematics and computers to understand principles behind observed chemical behavior

Notes Chemical- any substance that has a definite composition Not just the bad stuff Basic research- carried out for the sake of increasing knowledge Discovery by accident Applied research- carried out to solve a problem Discovery for profit

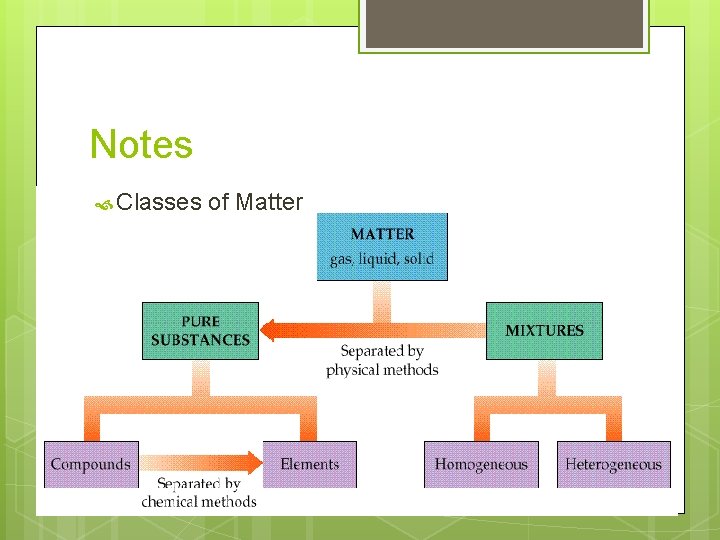
Notes Classes of Matter

Notes Matter- anything that takes up space and has mass Mass- NOT, I REPEAT NOT the same as weight!!! amount of matter in an object

Pure Substances (elements and compounds 1. Every sample of a given pure substance has exactly the same characteristic properties. a. Boiling point b. Freezing point c. Melting point d. Density Ex: water always has a boiling point of 100 C, melting point of 0 C, freezing point of 0 C and a density of l. 00 g/ml. Other pure substances have their own individual characteristics (bp, mp, fp, density) 2. Every sample of a given pure substance has exactly the same composition.

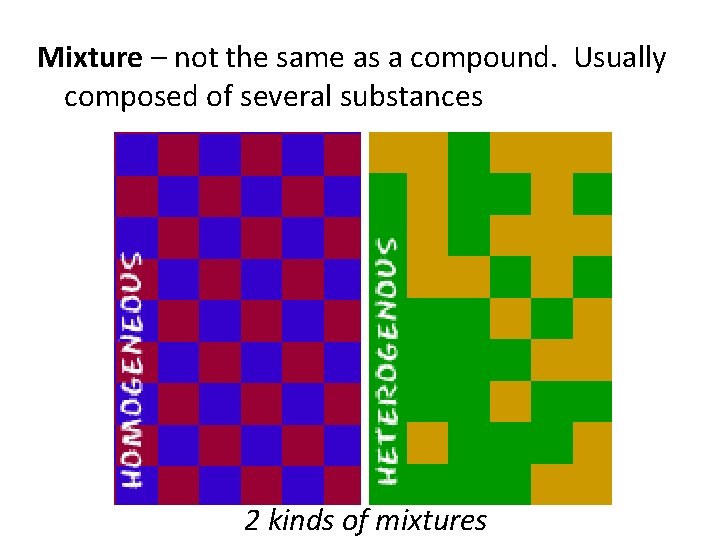
Mixture – not the same as a compound. Usually composed of several substances 2 kinds of mixtures

Opener • Copy down the question and the answer

Review • What is matter? • What are the two MAIN classifications of matter? • What are the two pure substances? • What are the two types of mixtures? Explain them

1. homogeneous mixtures – also called solutions and have the SAME composition throughout. Ex: milk, salt water

a. solute – the substance that gets dissolved. (What you have the least of) Example: salt b. solvent – the substance that does the dissolving. (What you have the most of. ) Example: water

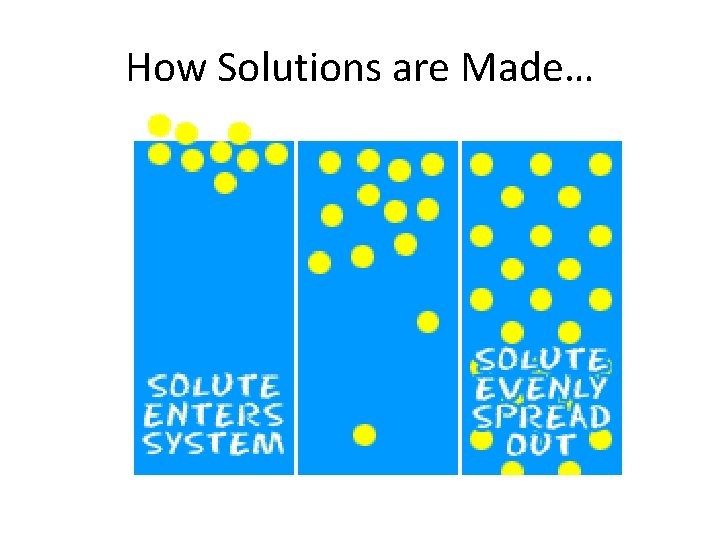
How Solutions are Made…

2. heterogeneous mixtures – the composition is NOT THE SAME throughout; oil and vinegar, blue cheese salad dressing, etc.

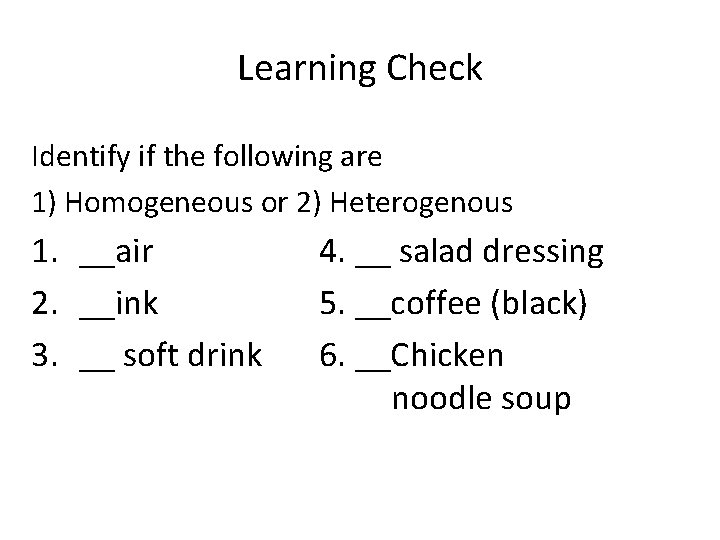
Learning Check Identify if the following are 1) Homogeneous or 2) Heterogenous 1. __air 2. __ink 3. __ soft drink 4. __ salad dressing 5. __coffee (black) 6. __Chicken noodle soup

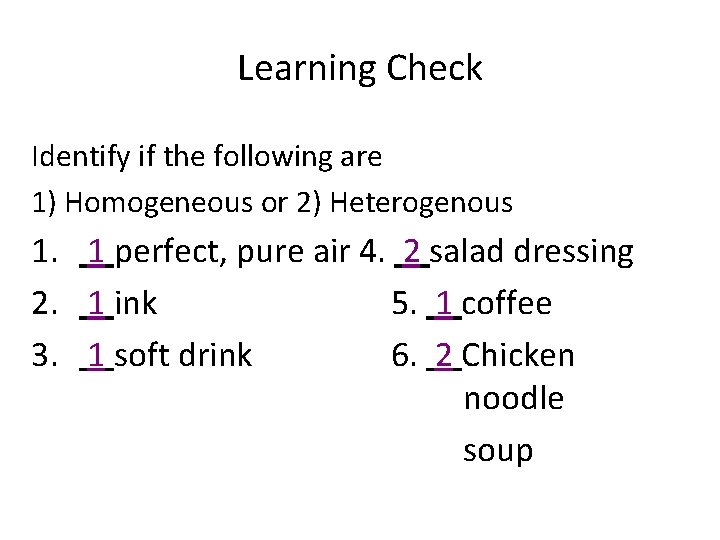
Learning Check Identify if the following are 1) Homogeneous or 2) Heterogenous 1. 1 perfect, pure air 4. 2 salad dressing 2. 1 ink 5. 1 coffee 3. 1 soft drink 6. 2 Chicken noodle soup

Learning Check Circle the solute and underline the solvent 1. 50 g of water and 5 g Na. Cl 2. 2. 20 g copper and 50 g zinc 3. 30%water and 70% alcohol 4. 18. 0 L of nitrogen & 12. 0 L of oxygen

Circle the solute and underline the solvent 1. 50 g of water and 5 g Na. Cl 2. 20 g copper and 50 g zinc 3. 30%water and 70% alcohol 4. 18. 0 L of nitrogen & 12. 0 L of oxygen

Practice Activity Card Sort In groups of 2, sort the card cutouts you have into two columns Column A should start with cards 1 and 2 Column B should start with cards 3 and 4 Place remaining cards into the correct column Gallery. Walk One stays to explain, one moves Can you group them different ways?

Homework Worksheet Answer On all parts of the worksheet the back… Growth Mindset Create 20 other examples of material to be classified Both are due TOMORROW!!!