Solutions Details Making a solution u Solvent solute








- Slides: 12

Solutions Details

Making a solution u Solvent, solute, and something to contain both. u Factors that affect solvation – Agitation – Temperature of solvent/solution – Surface area of solute

Degrees of saturation u Saturated: holds maximum amount of solute in given solvent at constant temperature and pressure u Solubility: the amt. of solute that dissolves in a given solvent at a specified temperature and pressure to give a saturated solution – Frequently given as grams/liter

More on saturation u If a solution contains less than the saturation amount of solute, it is called unsaturated u If you have a saturated solution, and cool if slowly, you can produce a supersaturated solution, which hold more solute than it “should” be able to. Crystallization will occur if a suitable seed is provided.

Liquid Solutions u Two liquids are miscible if they dissolve in each other in all proportions – The liquid in higher amount is considered the solvent u Liquids that are insoluble in each other are immiscible

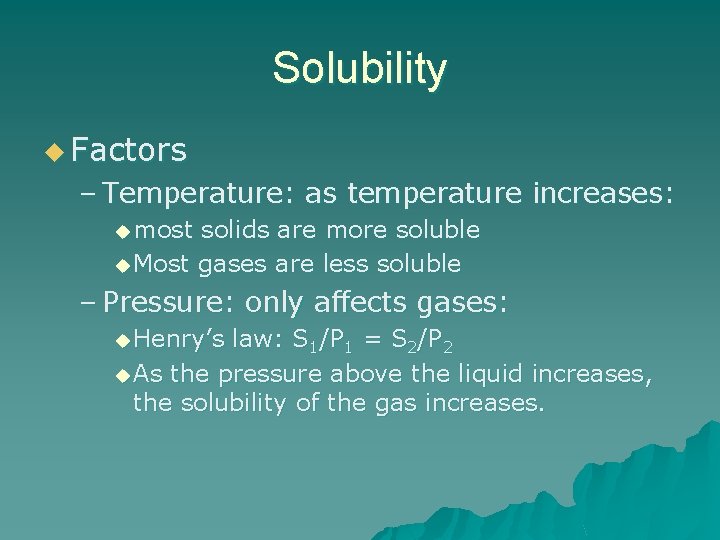
Solubility u Factors – Temperature: as temperature increases: u most solids are more soluble u Most gases are less soluble – Pressure: only affects gases: u Henry’s law: S 1/P 1 = S 2/P 2 u As the pressure above the liquid increases, the solubility of the gas increases.

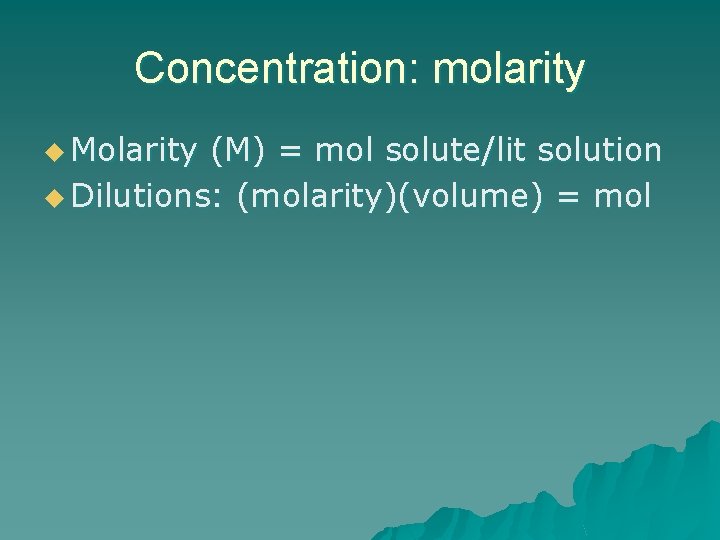
Concentration: molarity u Molarity (M) = mol solute/lit solution u Dilutions: (molarity)(volume) = mol

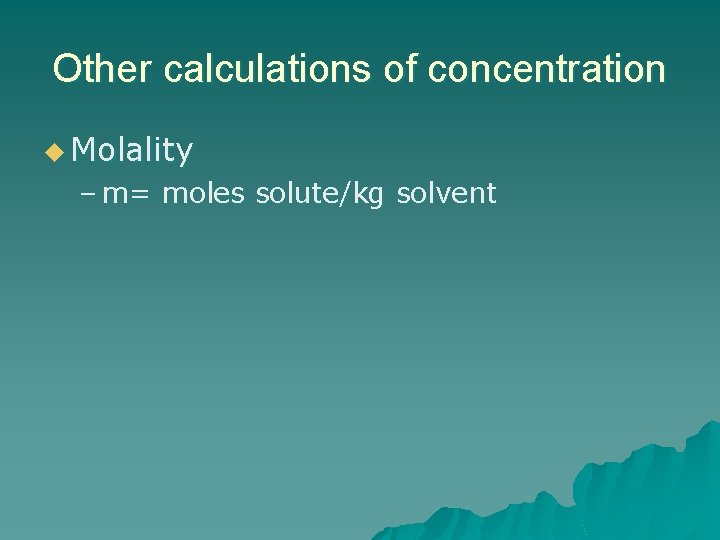
Other calculations of concentration u Molality – m= moles solute/kg solvent

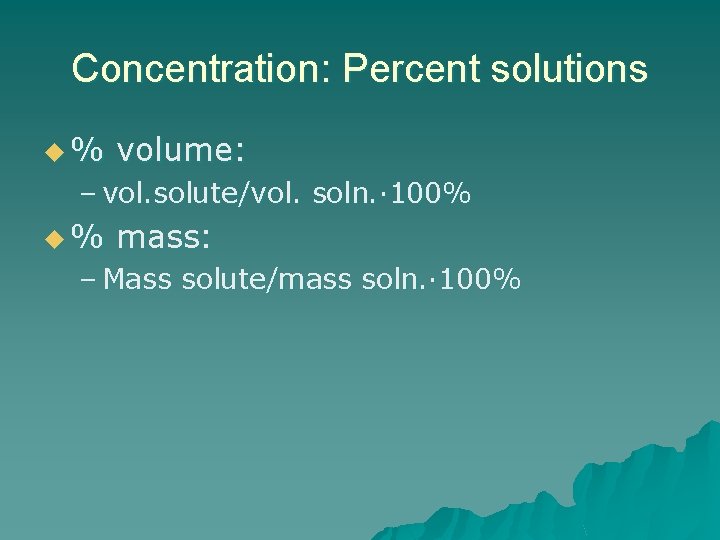
Concentration: Percent solutions u% volume: – vol. solute/vol. soln. · 100% u% mass: – Mass solute/mass soln. · 100%

Colligative Properties of Solutions u Vapor-pressure reduction – This occurs because the solute causes there to be different intermolecular attractions than would exist in pure solvent…ionic compounds, which dissociate, have a greater effect than non-dissociating solutes, such as sugar. – The decrease in vapor pressure is proportional to the amt. of solute present in the solution

Colligative Properties of Solutions u Freezing-point depression – The amount of lowering of a freezing point due to the same disruption and/or attractions of a solute in a solvent – Again, proportional to amount – Think about the salting of roads and sidewalks!

Colligative Properties of Solutions u Boiling point elevation – Since the addition of a nonvolatile solute lowers the vapor pressure, this means that it takes more energy for the solution to reach the boiling point – Again, the magnitude of the elevation is proportional to the amt. of solute dissolved in the solution