Chapter 13 Solutions Solution Solvent Solute Solution a







- Slides: 18

Chapter 13 Solutions

Solution = Solvent + Solute • Solution – a homogenous mixture of two or more substances or components, with the major component being the solvent and the other minor components being solutes • Solvent - a substance that dissolves another substance • Or, the substance present in greater amount • Solute - a substance which is dissolved by another substance • Or, the substance present in lesser amount

Colligative properties • A property that depends on the amount of a particles in a solution • Independent of type of particle • Ions, atoms, molecules • Electrolytes generate multiple particles when dissolved

Practice • Assuming complete ionization upon dissolution • 1 mole Na 2 CO 3 = ? moles of ions • 2 moles of Ca. Cl 2 = ? moles of ions • 3 moles of Fe 3(PO 4)2 = ? moles of ions

Colligative properties and nonelectrolytes • 1 mole of non volatile nonelectrolyte will form 1 mole of dissolved particles • Sugars, ethylene glycol • Vapor pressure lowering • Freezing point depression • Boiling point elevation • Osmotic pressure

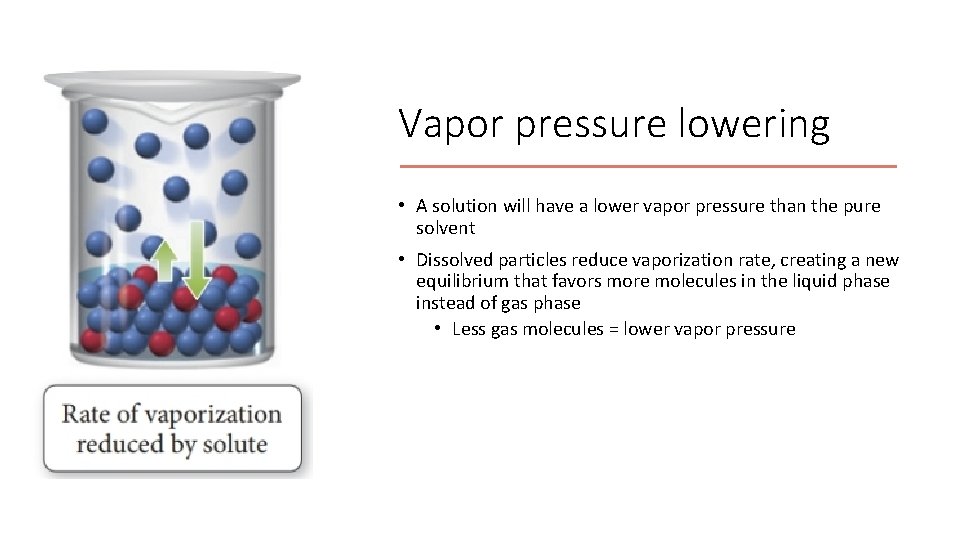
Vapor pressure lowering • A solution will have a lower vapor pressure than the pure solvent • Dissolved particles reduce vaporization rate, creating a new equilibrium that favors more molecules in the liquid phase instead of gas phase • Less gas molecules = lower vapor pressure

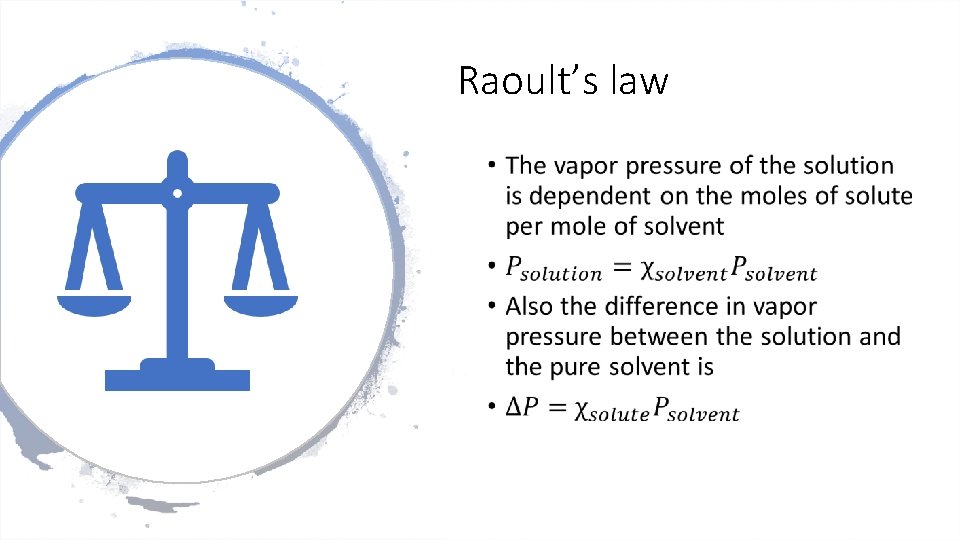
Raoult’s law •

Practice • Calculate the vapor pressure at 25°C of a solution containing 99. 5 g sucrose (C 12 H 22 O 11) and 300 g of water. The vapor pressure of pure water at 25°C is 23. 8 torr.

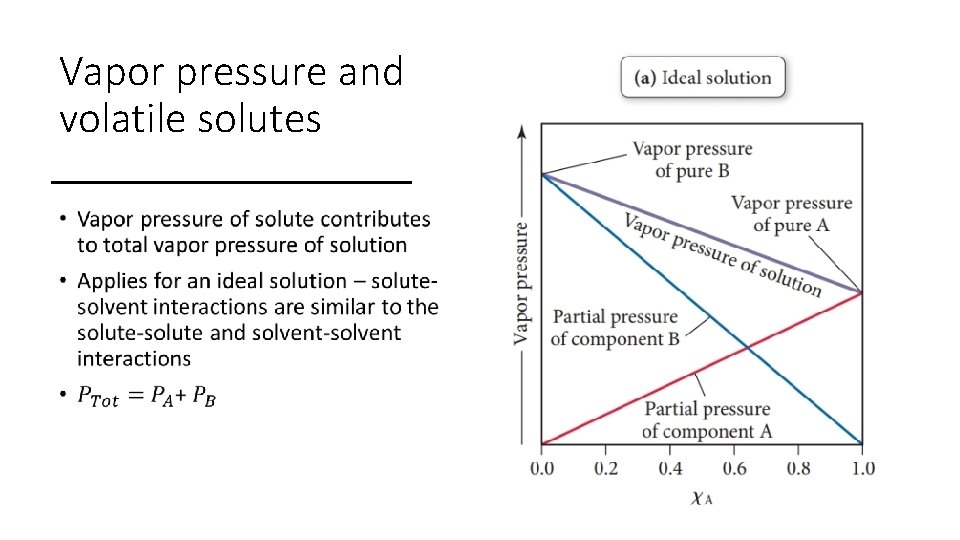
Vapor pressure and volatile solutes •

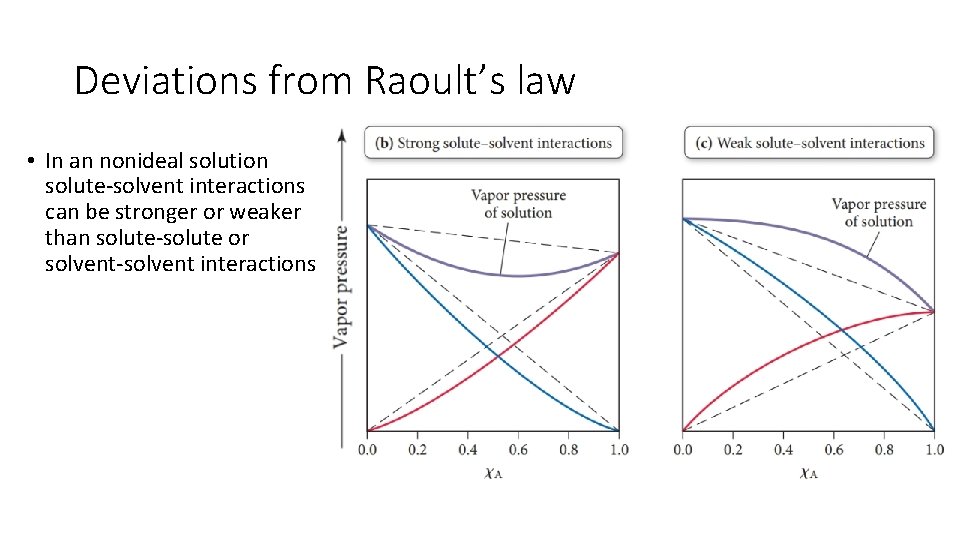
Deviations from Raoult’s law • In an nonideal solution solute-solvent interactions can be stronger or weaker than solute-solute or solvent-solvent interactions

Freezing point depression •

Boiling point elevation •

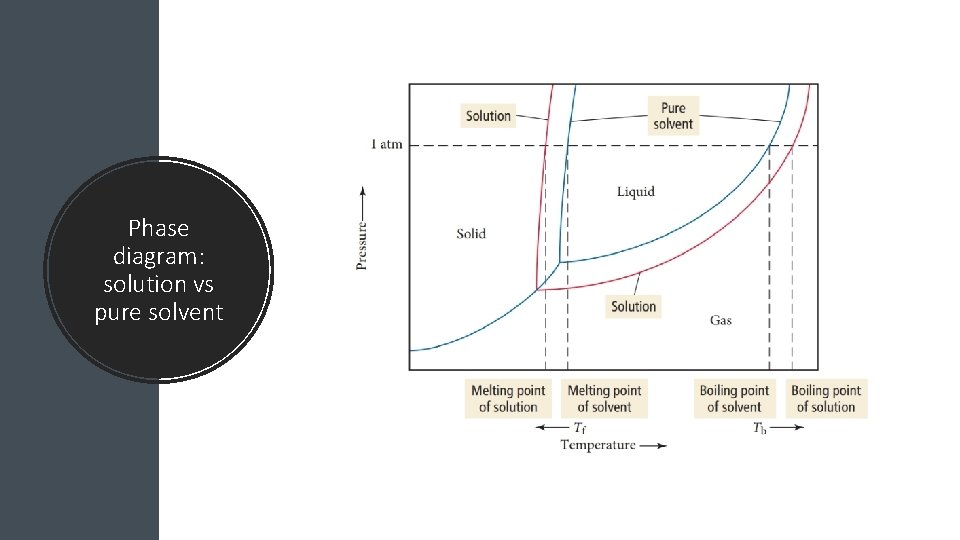
Phase diagram: solution vs pure solvent

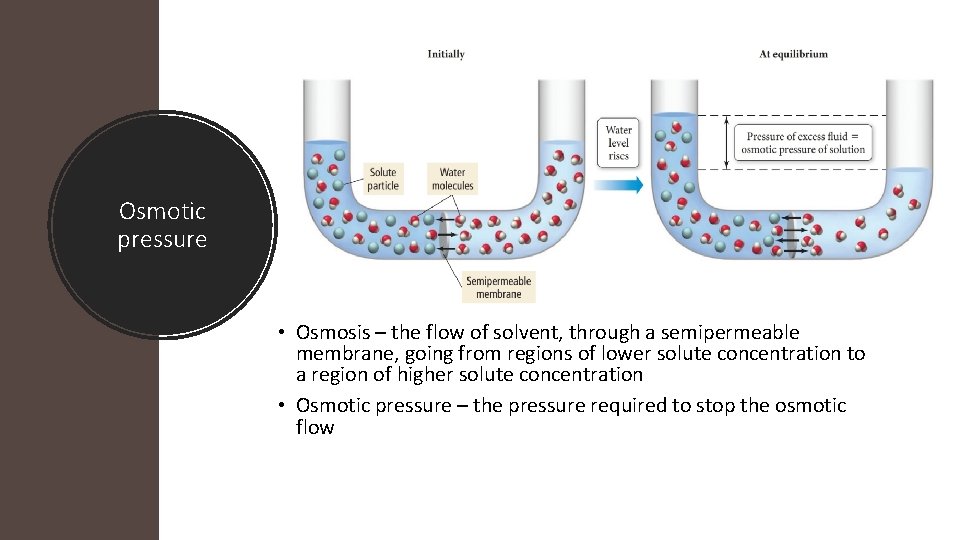
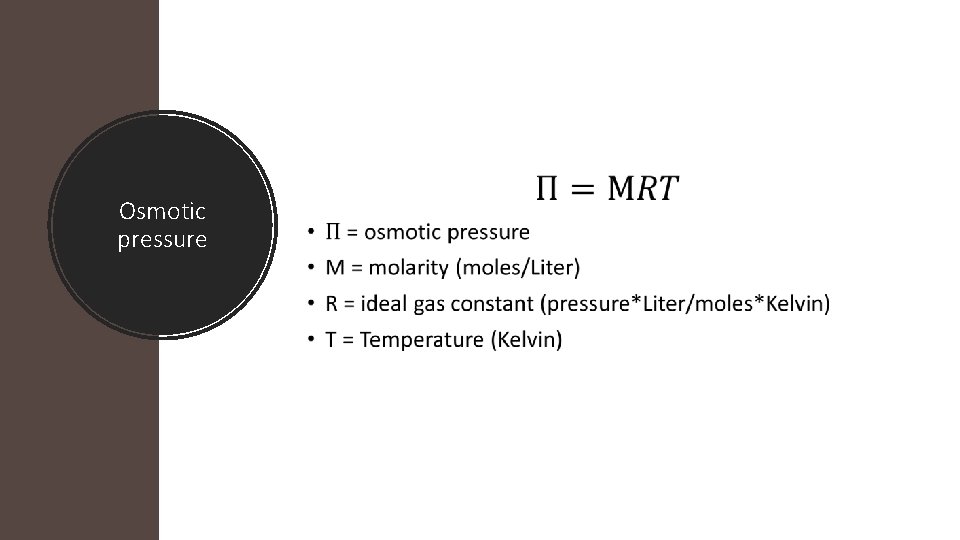
Osmotic pressure • Osmosis – the flow of solvent, through a semipermeable membrane, going from regions of lower solute concentration to a region of higher solute concentration • Osmotic pressure – the pressure required to stop the osmotic flow

Osmotic pressure •

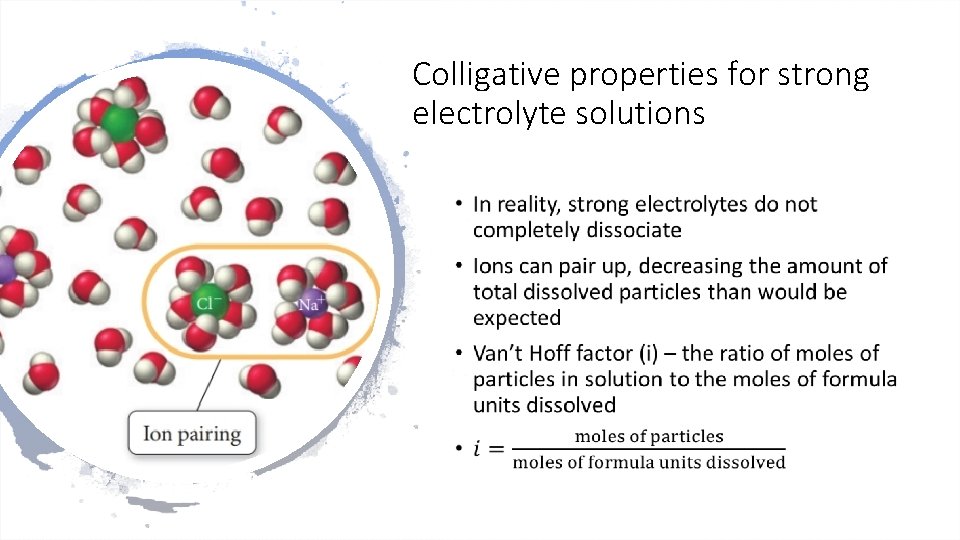
Colligative properties for strong electrolyte solutions •

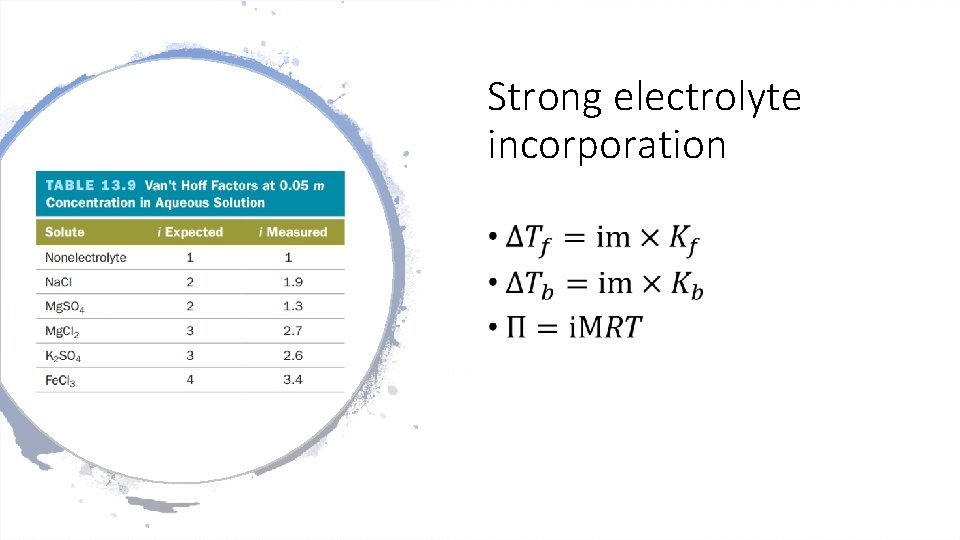
Strong electrolyte incorporation •

The end