1 Solutions Learning Outcomes Define solute solvent solution










- Slides: 15

1 Solutions – Learning Outcomes Define solute, solvent, solution. Examine and describe the difference between dilute, concentrated, and saturated solutions. Define solubility. Investigate solubility in water and the effect of temperature on solubility.

2 Solutions – Learning Outcomes Draw a solubility curve. Define crystallisation. HL: Grow crystals using alum or copper sulfate.

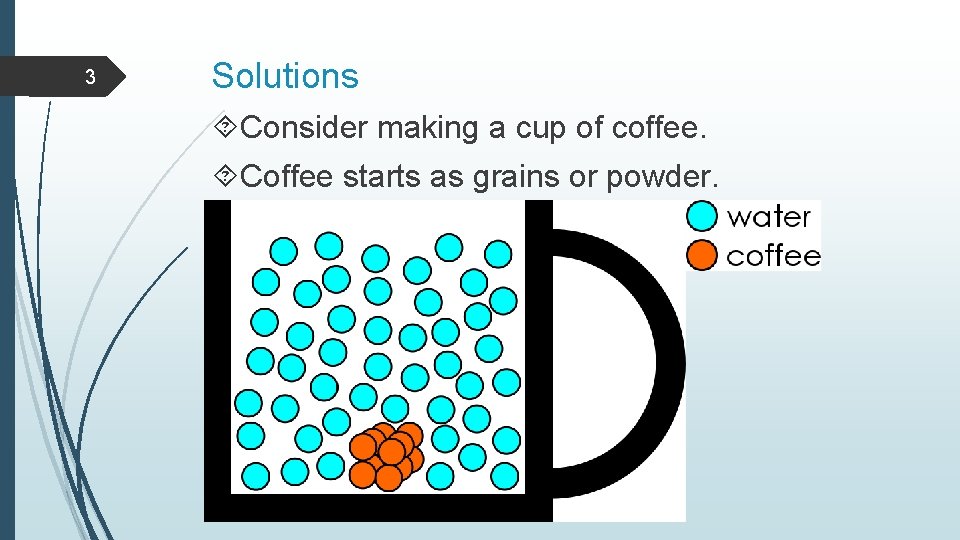
3 Solutions Consider making a cup of coffee. Coffee starts as grains or powder.

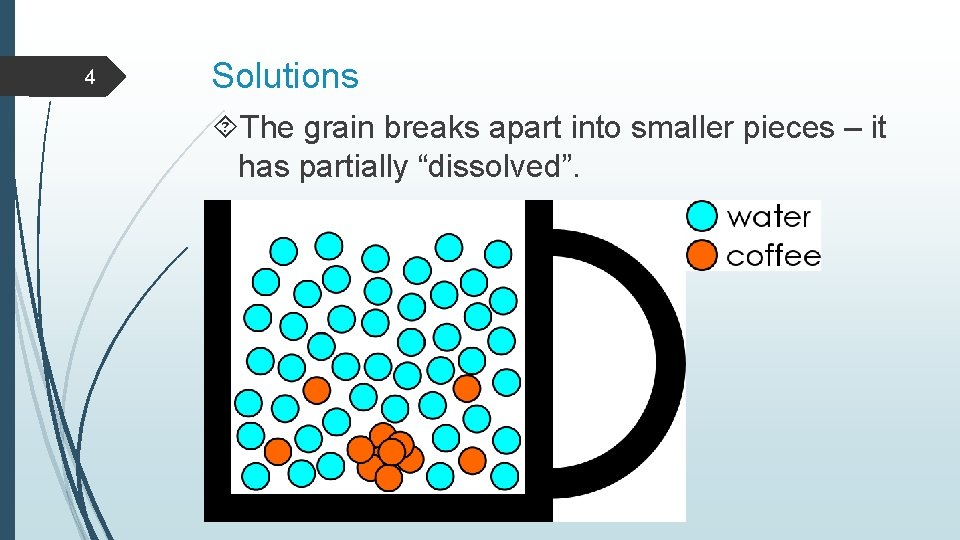
4 Solutions The grain breaks apart into smaller pieces – it has partially “dissolved”.

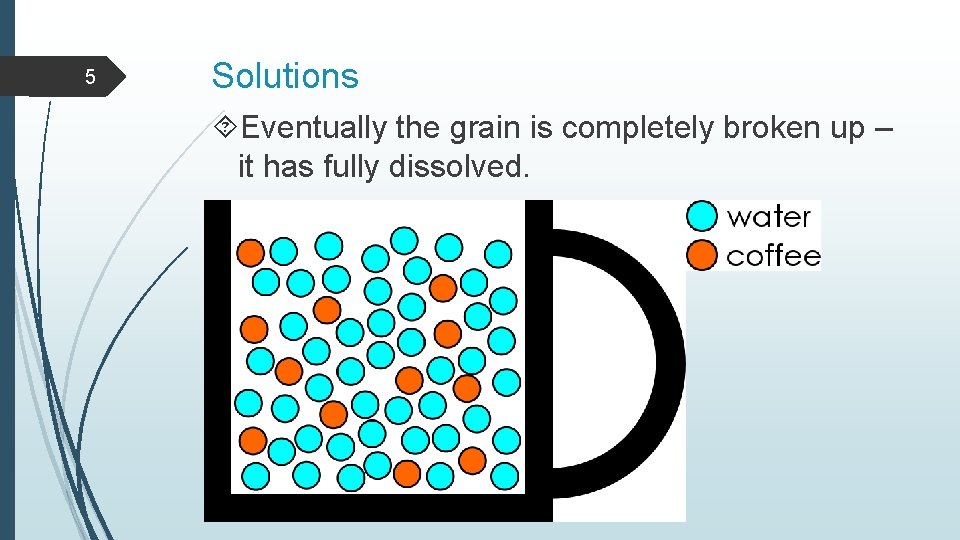
5 Solutions Eventually the grain is completely broken up – it has fully dissolved.

6 Solutions We say the coffee is a solute because it dissolves in the water. The water is a solvent because it dissolves the coffee. The mixture of coffee dissolved in water is called a solution.

7 Solutions A solvent is a substance that dissolves other materials to form a solution. A solute is a substance that dissolves in a solvent. A solution is a mixture of a solute and a solvent. If a material can dissolve in a solvent, it is said to be soluble (e. g. coffee in water). If a material cannot dissolve in a solvent, it is said to be insoluble (e. g. sand in water).

8 Solution examples

9 Solutions Which of these will dissolve in water? Cocoa powder Sand Dirt

10 Concentration Depending on how much solute is dissolved, we use different words to describe the resulting solution. Dilute solutions have little solute dissolved in a lot of solvent. Concentrated solutions have a lot of solute dissolved in a little solvent. Saturated solutions have as much solute dissolved as possible at that temperature.

11 Solubility How many spoons of sugar will dissolve in a cup of tea? Depends on: 1. volume of tea 2. temperature of tea We define solubility as being the amount of solute that will dissolve in a solvent at a particular temperature.

12 Effect of Temperature on Solubility usually increases with higher temperatures. e. g. at 70 o. C, you could dissolve more sugar in tea than you could at 20 o. C. One exception is oxygen in water – less oxygen can be dissolved in hot water than in cold water.

13 Solubility Curves Solubility curves are graphs of solubility vs. temperature. We plot solubility in g/100 g (amount of solute in 100 g of solvent) on the y-axis and temperature on the x-axis. This question comes up a whole lot in exams, learn it really well!

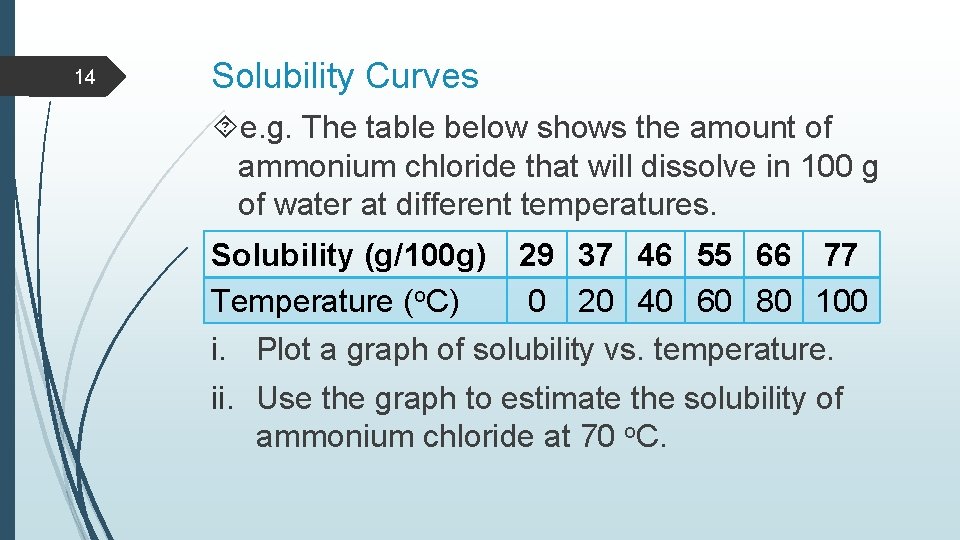
14 Solubility Curves e. g. The table below shows the amount of ammonium chloride that will dissolve in 100 g of water at different temperatures. Solubility (g/100 g) Temperature (o. C) 29 37 46 55 66 77 0 20 40 60 80 100 i. Plot a graph of solubility vs. temperature. ii. Use the graph to estimate the solubility of ammonium chloride at 70 o. C.

15 Crystallisation is the formation of crystals by cooling a saturated solution. Since colder solutions can be less saturated, when you cool saturated solutions, some of the solute “undissolves” and forms crystals.