Solutions Homogeneous mixture Solute Solvent Solution Formation Rate






















- Slides: 23

Solutions • Homogeneous mixture • Solute • Solvent

Solution Formation Rate • Factors affecting it… – Temperature—think about dissovling sugar in tea – Agitation—again think about the tea – Particle size—granulated sugar vs. powdered sugar?

Henry’s Law • The solubility (C) of a gas in a liquid is directly proportional to the pressure above the liquid • C 1 = C 2 P 1 P 2 • Think about carbonated beverages

Henry’s Law Problem • At 20°C and 1. 00 atm, the solubility of oxygen gas in water is 0. 0448 g/L. What will the solubility be if the pressure is increased to 1. 75 atm? Answer: 0. 0784 g/L

Concentration • Qualitative descriptions do not give amounts of solute in solution. – Concentrated—lots of solute – Dilute—not much solute • Note: no quantities are provided

Concentration • A few types… – Molarity – Mass percent – Mass/volume percent – Volume/volume percent – Mole fraction – Molality

Molarity • Represented by M—always uppercase • M = #moles of solute #liters of solution • Units will be mol/L, or you may write it as M • A 2 M solution is described as “two molar”

Mass Percent • Represented as m/m% • m/m% = mass of solute x 100 mass of solution • Mass units must be the same for the solute and the solution • Unit-less

Mass/volume Percent • Represented as m/v% • m/v% = #g of solute x 100 #m. L of solution • Units are specific • g/m. L

Volume/volume Percent • Represented by (v/v)% • (v/v)% = volume of solute x 100 volume of solution • Volume units must be the same for the solute and the solution • Unit-less

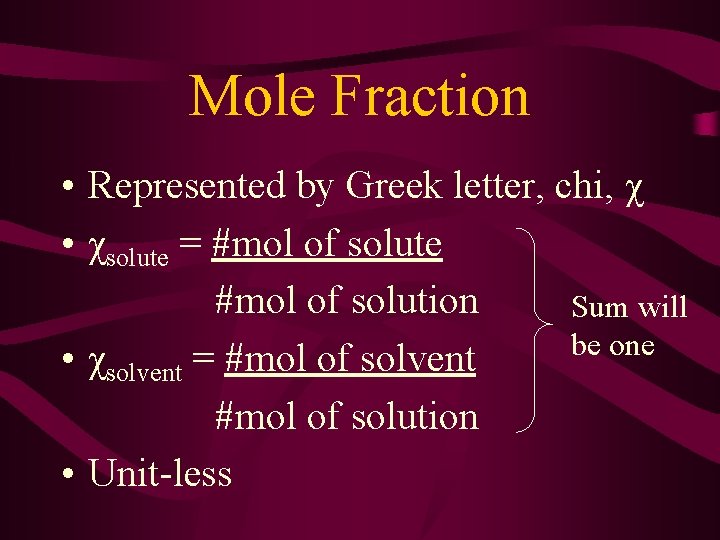
Mole Fraction • Represented by Greek letter, chi, χ • χsolute = #mol of solute #mol of solution Sum will be one • χsolvent = #mol of solvent #mol of solution • Unit-less

Molality • Represented by m—always lowercase • m = #moles of solute #kg of solvent • Units will be mol/kg, or you may write it as m • A 2 m solution is described as “two molal”

Dilutions • I don’t usually stock every concentration of every acid that I need…so I start with the most concentrated form and make whatever molarity (the most common measurement of concentration) I need. • (M 1)(V 1) = (M 2)(V 2)

Dilutions • The rule of thumb is if you’re making an acid dilution in a beaker, always add the acid to water…so if something splashes, it will be the water!

Practice #1 • How many grams of sodium hydroxide are required to make 250 m. L of a 0. 25 M solution? Answer: 2. 50 g Na. OH

Practice #2 • How many grams of water are present in a 5. 00%(m/m) solution containing 0. 875 g of calcium acetate? Answer: 16. 625 g H 2 O

Practice #3 • How many grams of acetic acid are present in 4. 50 L of a 5. 00%(m/v) solution? Answer: 225 g HC 2 H 3 O 2

Practice #4 • A 95% (v/v) solution of ethanol in double-distilled water is used to clean surfaces in a laboratory setting. If you have 500 m. L of the solution, how many of those m. L are water? Answer: 25 m. L H 2 O

Practice #5 • 10. 0 g of calcium acetate are added to 100 g of water. What are the mole fractions of both the solute and the solvent? Answer: χcalcium acetate = 0. 0114 χwater = 0. 989

Practice #6 • 10. 0 g of calcium acetate is added to 100 g of water. What is the molality of this solution? Answer: 0. 632 mol/kg or m

Practice #7 • If I need to make 250 m. L of 6 M sulfuric acid, and all I have in my cabinet is full strength, 18 M sulfuric acid, describe how I make the dilution. Answer: measure 83. 3 m. L of 18 M H 2 SO 4 and pour into the 250 -m. L volumetric flask. Fill the flask to the etched line with water, cork it, and agitate it.

A Doozie Problem • A solution is prepared by mixing 1. 00 g of ethanol, CH 3 CH 2 OH, with 100. 0 g of water to give a final volume of 101. 0 m. L of solution. Calculate the molarity, mass percent, mass/volume percent, molality, mole fraction of the ethanol, and mole fraction of the water.

A Doozie’s Answers… • molarity = 0. 215 M or 0. 215 mol/L • mass percent = 0. 990% • mass/volume percent = 0. 990(g/m. L)% • molality = 0. 217 m or 0. 217 mol/kg • mole fractionethanol = 0. 00389 • mole fractionwater = 0. 996