Solutions are homogeneous mixtures solute solvent Solute is






- Slides: 8

* *Solutions are homogeneous mixtures (solute + solvent). *Solute is the dissolved substance. * Seems to “disappear” in the solvent. *Solvent is the substance in which the solute dissolves. * Does not appear to change state. *Aqueous solutions * Have water as the solvent. * Water is called the universal solvent because it dissolves so many substances. *Metal solutions (one metal dissolved in another) are called alloys (ex: steel, brass, bronze). 1

* * When one substance (the solute) dissolves in another (the solvent) it is said to be soluble. * Ex: salt and ethanol are both soluble in water * When one substance does not dissolve in another it is said to be insoluble. * Ex: oil is insoluble in water * When a solute and solvent have the same state (gas, liquid, or solid), the solvent is the component present in the highest percentage. * Metal solutions (one metal dissolved in another) are called alloys. *Ionic Compounds * Dissociate into ions when dissolved in water * Polar water molecules interact with positive and negative ions to dissolve the compound-ions surrounded by water molecules are hydrated 2

*Covalent Compounds *Polar covalent compounds have “polar” groups that tend to be soluble in water. * Ethanol (a liquid) or sucrose (table sugar), for example, have polar O-H bonds that interact with water to enhance solubility. *Nonpolar covalent compounds have molecules that do not form attractions to water molecules because all their bonds are essentially non-polar, which prevents them from being soluble in water. * Petroleum products or vegetable oil molecules are examples. 3

*Molecules that are similar in structure tend to form solutions: “like dissolves like” *A given solvent dissolves solutes that have polarities similar to its own. *Water (a polar molecule) dissolves most polar solutes (liquids or solids). *Non-polar solvents dissolve nonpolar solutes. Gasoline, for example, dissolves in oil since they are both nonpolar compounds. However, neither gasoline nor oil will dissolve in water because water is polar. *Gases are always soluble in each other. 4

*Concentration = amount of solute in a given amount of solution. *A concentrated solution has a high proportion of solute to solution. *A dilute solution has a low proportion of solute to solution. *A saturated solution contains the maximum amount of solute that will dissolve in the solvent at a particular temperature. (The higher the temperature the greater the saturation. ) *An unsaturated solution contains less solute than the saturation limit. *A supersaturated solution contains more solute than the saturation limit. * Unstable; the solute easily precipitates out of solution. 5

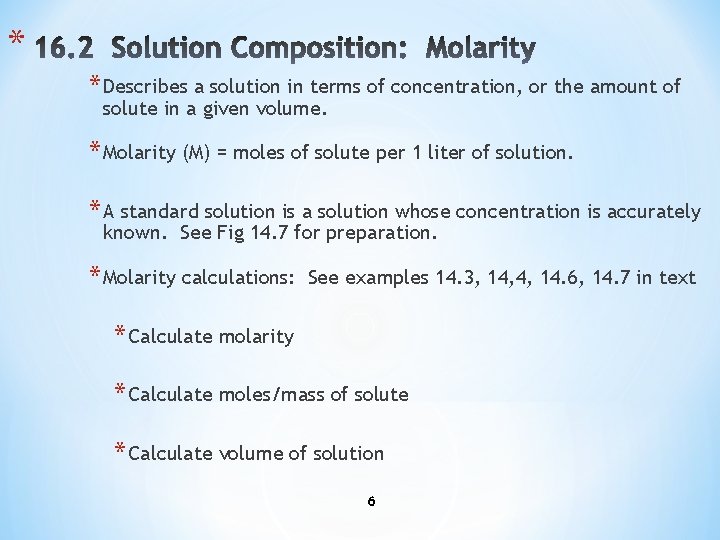
* * Describes a solution in terms of concentration, or the amount of solute in a given volume. * Molarity (M) = moles of solute per 1 liter of solution. * A standard solution is a solution whose concentration is accurately known. See Fig 14. 7 for preparation. * Molarity calculations: See examples 14. 3, 14, 4, 14. 6, 14. 7 in text * Calculate molarity * Calculate moles/mass of solute * Calculate volume of solution 6

* *Dilution is adding solvent to decrease the concentration of a solution. *The amount of solute stays the same, but the solute concentration decreases due to the added solvent. *Dilution Formula: M 1 x V 1 = M 2 x V 2 M 1 V 1 = before dilution; M 2 V 2 = after dilution *Since molarity is defined in terms of liters, change any volume in milliliters to solve dilution problems. *A standard solution is one whose concentration is accurately known. It can be used to make dilutions. 7

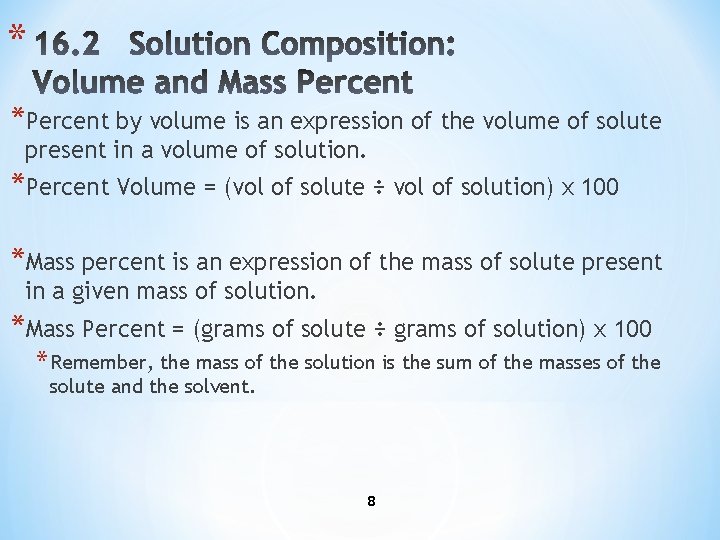
* *Percent by volume is an expression of the volume of solute present in a volume of solution. *Percent Volume = (vol of solute ÷ vol of solution) x 100 *Mass percent is an expression of the mass of solute present in a given mass of solution. *Mass Percent = (grams of solute ÷ grams of solution) x 100 * Remember, the mass of the solution is the sum of the masses of the solute and the solvent. 8