GRADE 7 SCIENCE Solutions Solutions a review A










































- Slides: 54

GRADE 7 SCIENCE Solutions

Solutions… a review �A homogeneous mixture � They appear as ONE substance Examples: tap water, vinegar, gold jewelry

We will learn: � identify the solute and the solvent in a variety of solutions � distinguish between soluble and insoluble substances � describe the concentration and solubility of substances qualitatively and quantitatively

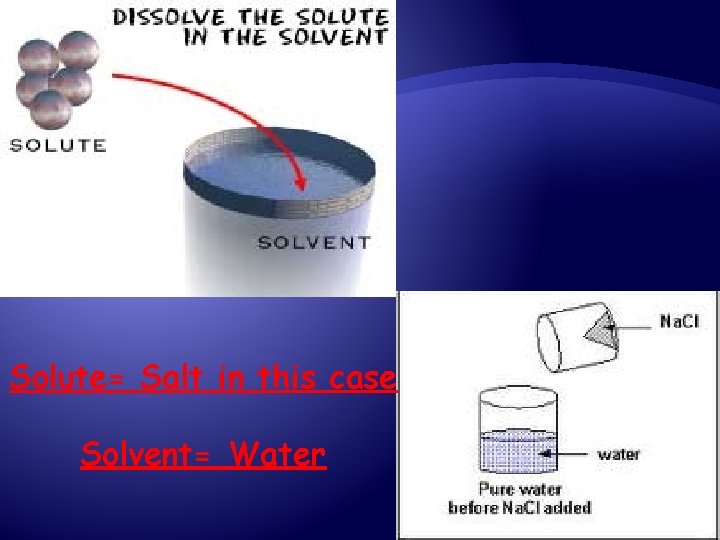
Solutions have 2 parts: Solute The substance that dissolves (found in less amounts) Solvent The substance in which the solute dissolves (found in the greatest amounts)

Solute= Salt in this case Solvent= Water

Page 255: Solute or Solvent? � A. Brass is used to make many objects. Brass is a solution of zinc metal in a copper metal. � B. Hydrogen peroxide is used to disinfect cuts. Hydrogen peroxide is a solution of 3% hydrogen and 97% water.

Solute or Solvent? � C. Dental amalgam is a metal that is used to fill cavities. It is a solution of mercury in tin. � D. Deicing fluid is a cleaner used to clean car and truck windshields. It is made up of a solution of propylene glycol in water.

Solutions song!- Journey with Me! http: //www. youtube. com/watch? v=3 G 472 AA 3 SEs

Dissolving. . . �To mix completely (the solute dissolves into the solvent. ) �Some substances are able to dissolve better than others.

For those about to dissolve- we solute you! http: //www. youtube. com/watch? v=VTmf. QUNLl. MY

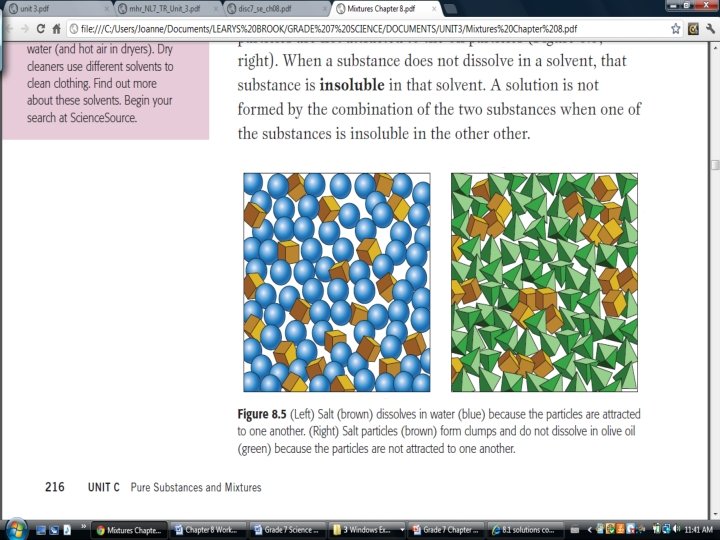
How are substances soluble? �The Particle Theory of Matter states that “there attractive forces between the particles”.

�In order to dissolve, the particles must be more strongly attracted to the particles of the solvent than to themselves. �This means that the solute is soluble in that solvent.

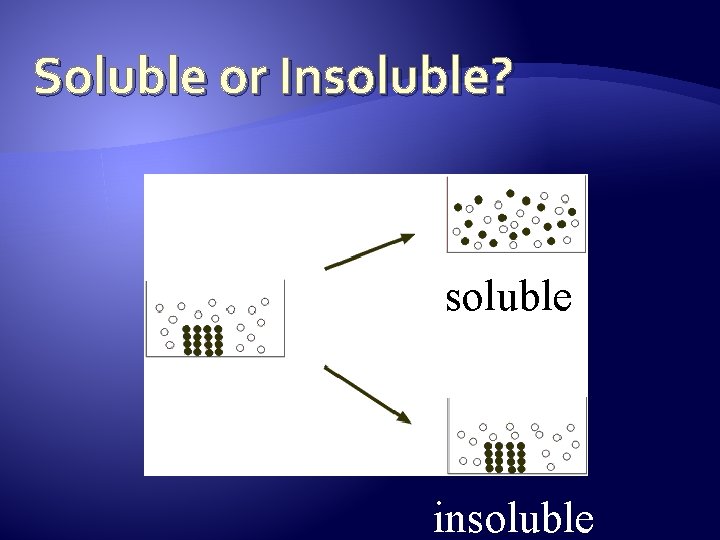
� If something is ABLE to dissolve then it is soluble. � For example, sugar dissolves in water.

Particle Theory- Dissolving animation

How are substances insoluble? �If the particles of the solute are more attracted to their own particles than the solvent particles, dissolving does NOT occur. The solute is said to be insoluble in that solvent. (ie. Mechanical)

� If something is UNABLE to dissolve then it is insoluble. � For example, sand does not dissolve in water.


Soluble or Insoluble? soluble insoluble

Solvent or Not? Some materials are good solvents for some solutes but not others. For example, oil is insoluble in water but soluble in gasoline.

States of Solutes and Solvents- Put book and workbook away. Hand out exit cards. Below is an example! Air Solute: oxygen, carbon dioxide, other gases Gas Solvent: nitrogen (most of our air is actually Nitrogen!!) Gas

Soda: (a combination of carbon dioxide in water). Identify water or carbon dioxide as the solute/solvent and say which state they are. Solute: ? Solvent: ? State of Solute: ? State of Solvent: ?

Vinegar: (a combination of 3% acetic acid and 97% water). Identify water or acetic acid as the solute/solvent and say which state they are. Solute: ? Solvent: ? State of Solute: ? State of Solvent: ?

Filtered Sea Water: (salt in water). Identify water or salt as the solute/solvent and say which state they are. Solute: ? Solvent: ? State of Solute: ? State of Solvent: ?

Brass (an alloy of a zinc metal in a copper metal) Identify zinc or copper as the solute/solvent and say which state they are. Solute: ? Solvent: ? State of Solute: ? State of Solvent: ?

Grade 7 Science Concentrations of Solutions

Concentration (p 4 of workbook; 264) The two cups below each had hot water with a tea bag sitting in them. The time below the cup indicates how long the tea bag has been in the cup. 10 minutes 2 minutes

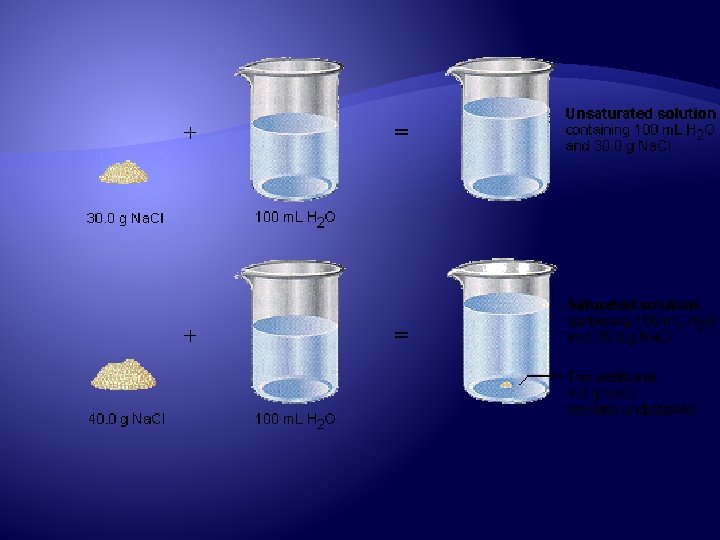
Concentration is… The quantity of solute that is dissolved in a certain quantity of the solvent. Can be described qualitatively or quantitatively.

Qualitative: Using words such as like “dilute” or “concentrated”

Quantitative: �Using numbers. This is especially important when safety is an issue!

Student Activity… 1. Demo of qualitative concentration 2. Demo of quantitative concentration

Student Practice… Quantitative? #1(a). Food coloring made the water blue. Qualitative (b). Adding 3 m. L of food coloring turned 250 m. L of water blue. Quantitative

#2(a). The water became warmer. Qualitative (b). The water’s temperature increased by 5 degree Celsius. Quantitative

#3(a). We needed just over a dozen floor tiles for our model room. Qualitative (b). We needed 14 floor tiles for our model room. Quantitative

#4(a). The liquid boiled in 5 min. Quantitative (b). The liquid took only a few minutes to boil. Qualitative

#5(a). The mass of this solid is 5 g more than that Quantitative one. (b). This solid is heavier Qualitative than that one.

#6(a). He drinks eight glasses of water each day. Qualitative (b). He drinks 2 L of water each day. Quantitative

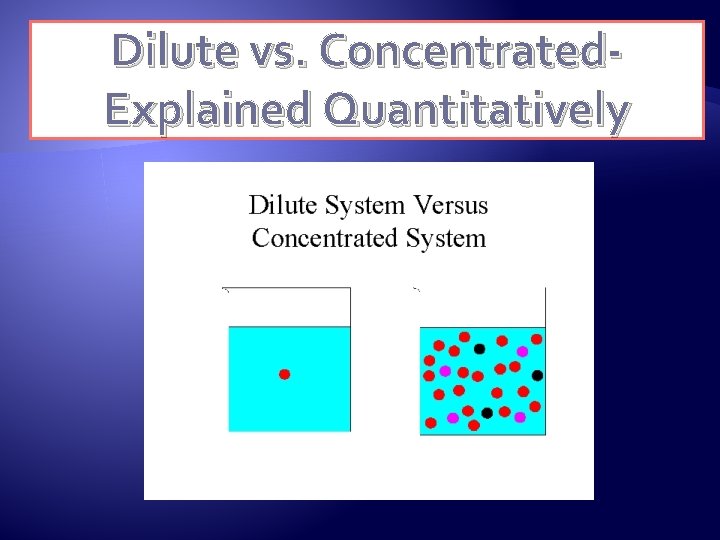
Dilute vs. Concentrated Dilute �There is a small mass of dissolved solute for a certain quantity of solvent. Concentrated �There is a large mass of dissolved solute for a certain quantity of solvent

Dilute vs. Concentrated- Explained Qualitatively

Dilute vs. Concentrated- Explained Quantitatively

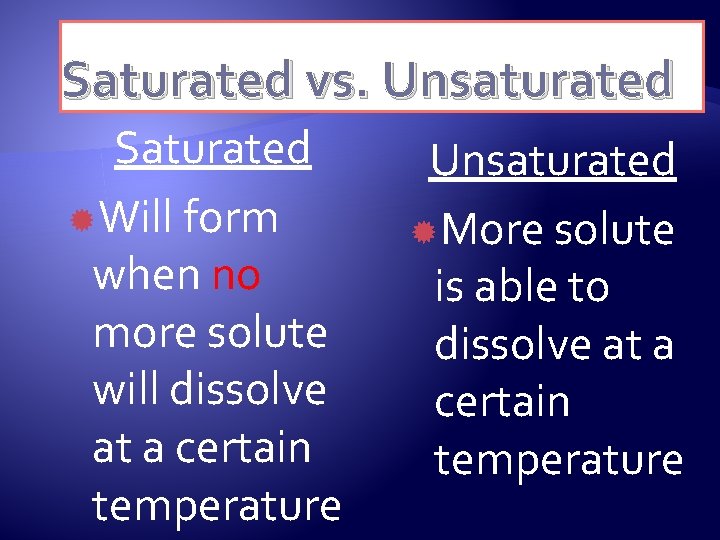
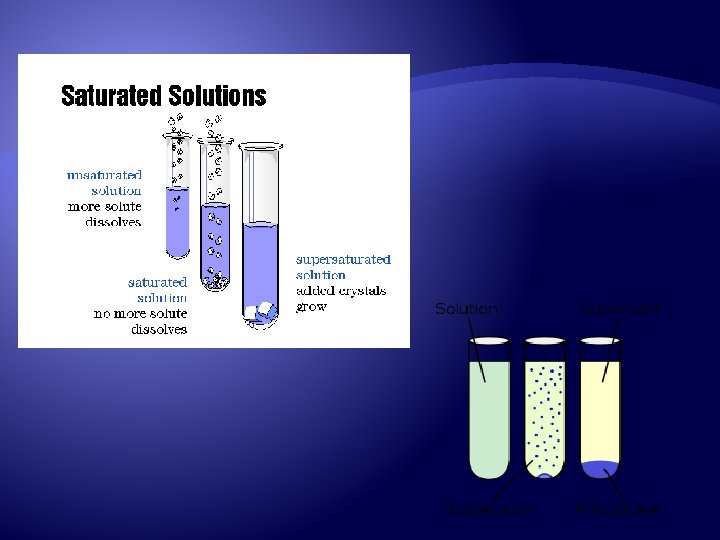
Saturated vs. Unsaturated Saturated Will form when no more solute will dissolve at a certain temperature Unsaturated More solute is able to dissolve at a certain temperature



Question to ponder… Can a solution be considered to be concentrated be unsaturated? Explain

Question to ponder… 1. If there are two glasses on Kool Aid on the table, one is unsaturated the other is saturated. How would you be able to tell the difference between the two?

Question to ponder… 2. It is possible to “unsaturate a saturated solution”. Suggest ways you might be able to do this. (Hint: How could you make more sugar dissolve in a glass of water? )

GRADE 7 SCIENCE Solutions and Solubility

How Does Temperature Affect Solubility? Complete Core Activity 8 -2 A page 268 -9

Rate of Dissolving… Factors that affect the rate of dissolving include: Size of the solute Temperature Pressure

Size of Solute The smaller the solute particles, the quicker they will dissolve faster

Temperature The higher the temperature, the more solute and the faster the solute will dissolve faster

Pressure Gases are more soluble in liquids under higher pressure (see page 267) Opened bottle decreases pressure and “bubbles” come out of solution

Quantitative Description Expressed as the amount of solute per unit volume. Examples: g/L g/m. L ppm (parts per million) %

Converting g/m. L to g/L **Remember there are 1000 m. L in 1 L. 1 g/m. L = ? g/L 1 x 1000 = 1000 therefore 1000 g/L

Practice Problems… 0. 3 g/m. L = ? g/L 8. 9 g/m. L = ? g/L 300 g/ L 8900 g/ L