Acids and Bases Solutions Draw on your own

Acids and Bases Solutions
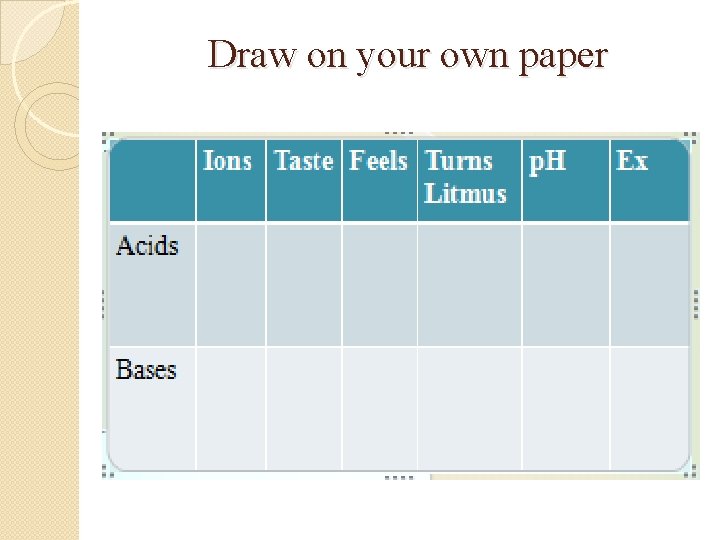
Draw on your own paper

Use these words to fill in your chart �Bitter �Blue �H+ �Lemons �OH�Red Slippery Soap Sour Squeaky <7 >7
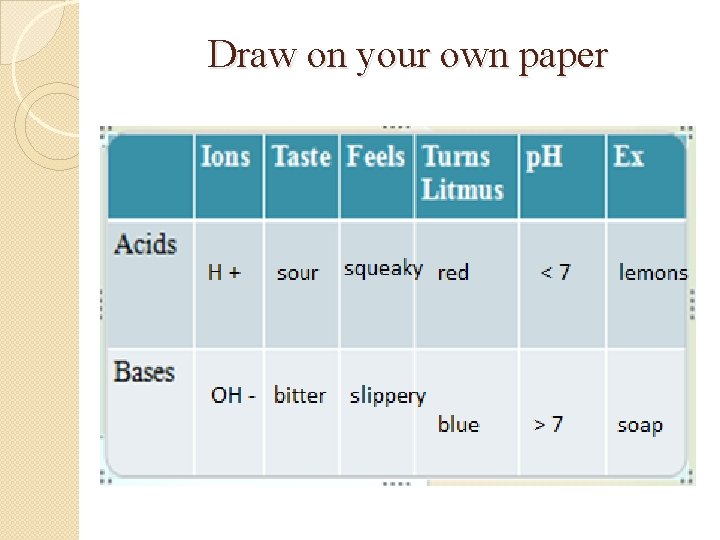
Draw on your own paper
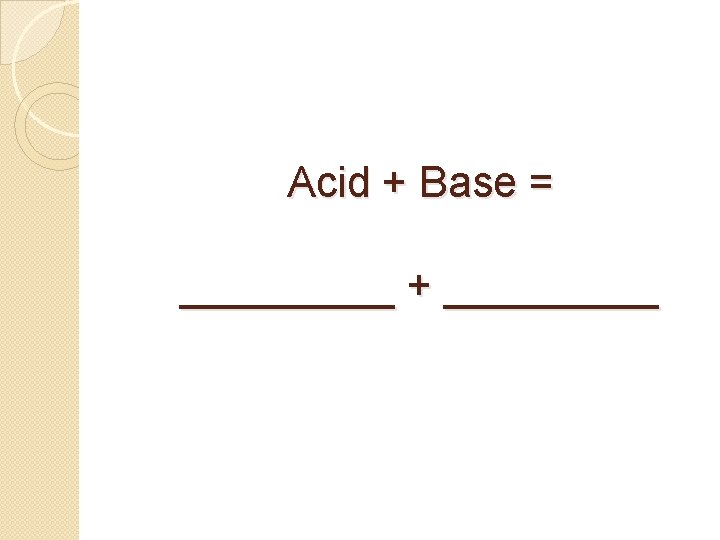
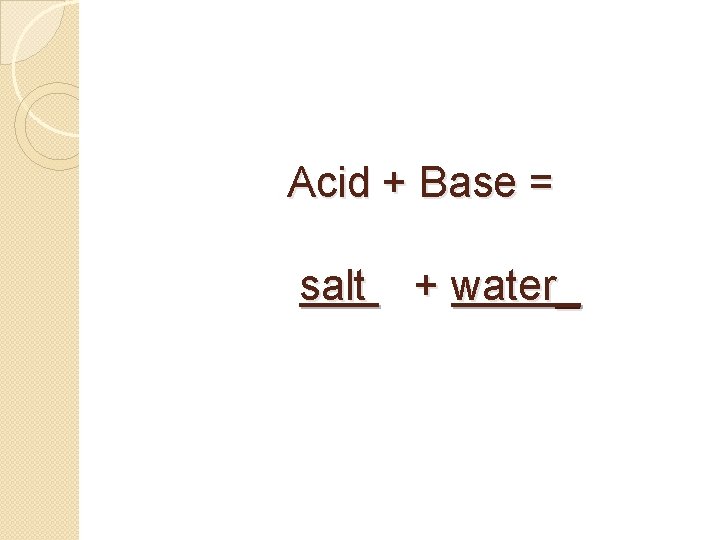
Acid + Base = salt + water_

Acid + Base is a __________ reaction.

Acid + Base is a neutralization reaction.
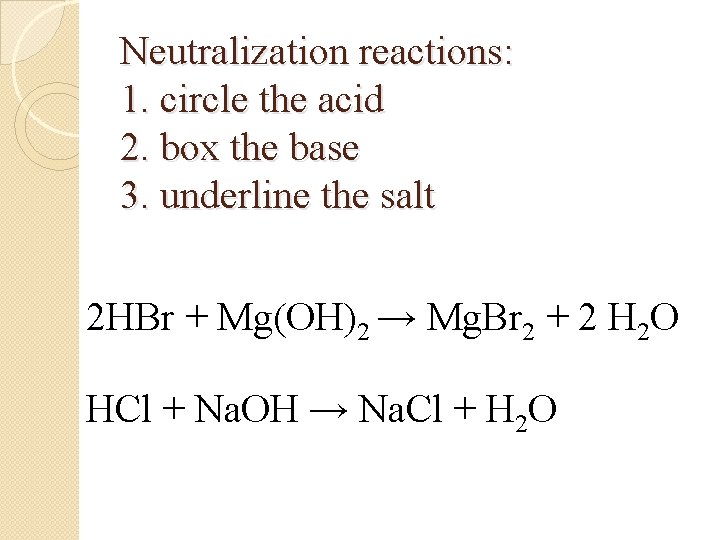
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt 2 HBr + Mg(OH)2 → Mg. Br 2 + 2 H 2 O HCl + Na. OH → Na. Cl + H 2 O
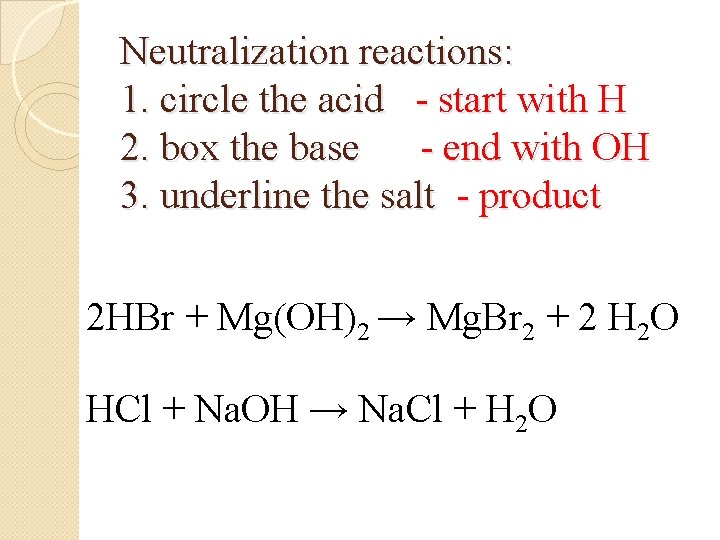
Neutralization reactions: 1. circle the acid - start with H 2. box the base - end with OH 3. underline the salt - product 2 HBr + Mg(OH)2 → Mg. Br 2 + 2 H 2 O HCl + Na. OH → Na. Cl + H 2 O
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt
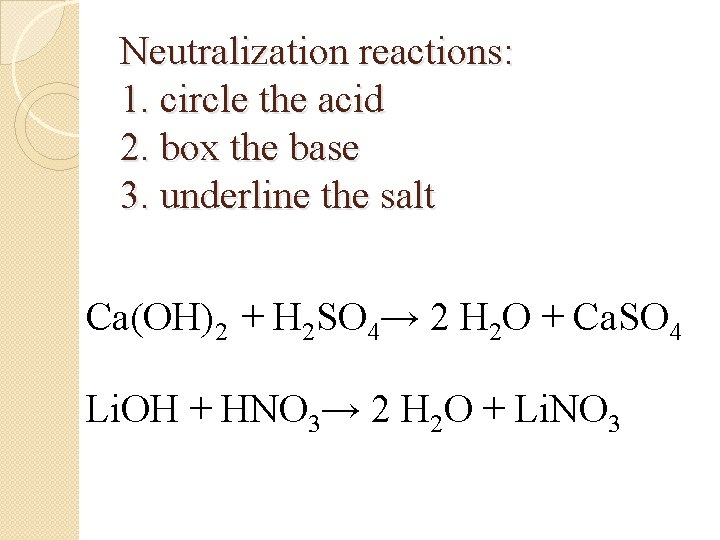
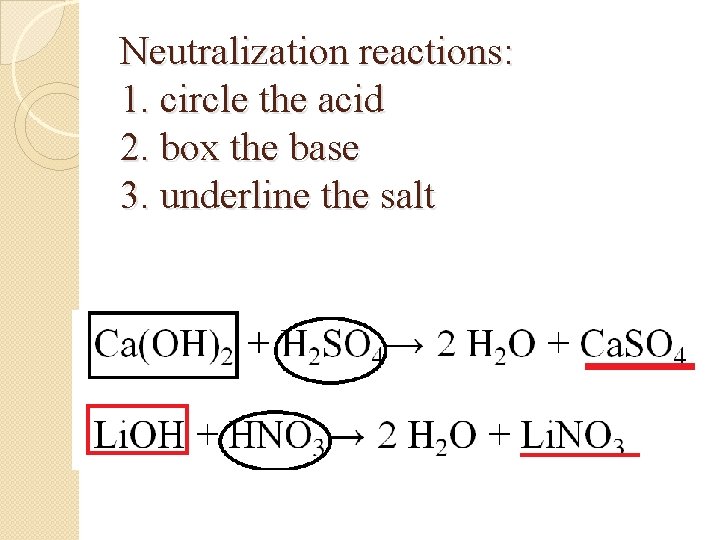
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt Ca(OH)2 + H 2 SO 4→ 2 H 2 O + Ca. SO 4 Li. OH + HNO 3→ 2 H 2 O + Li. NO 3
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt
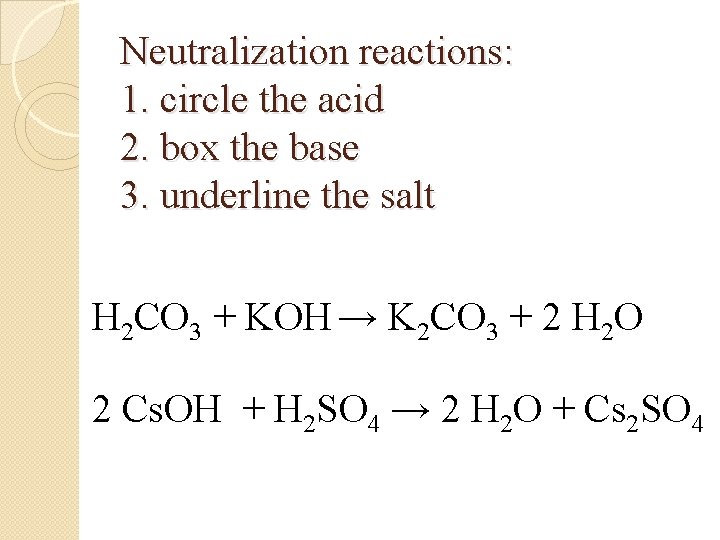
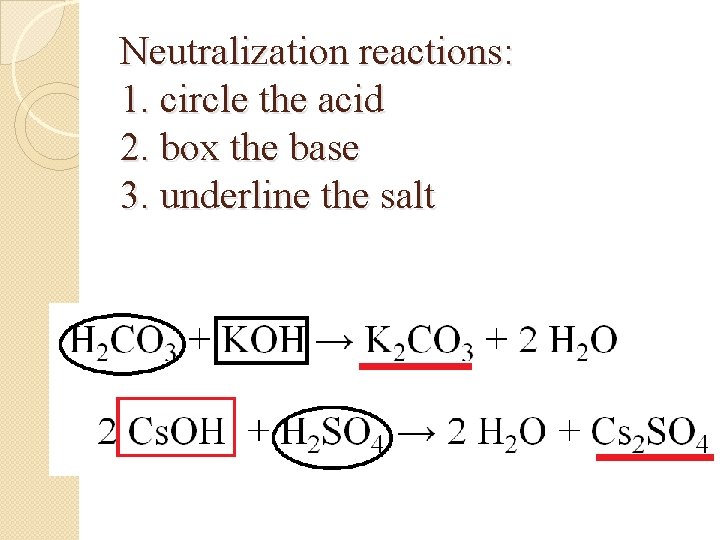
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt H 2 CO 3 + KOH → K 2 CO 3 + 2 H 2 O 2 Cs. OH + H 2 SO 4 → 2 H 2 O + Cs 2 SO 4
Neutralization reactions: 1. circle the acid 2. box the base 3. underline the salt
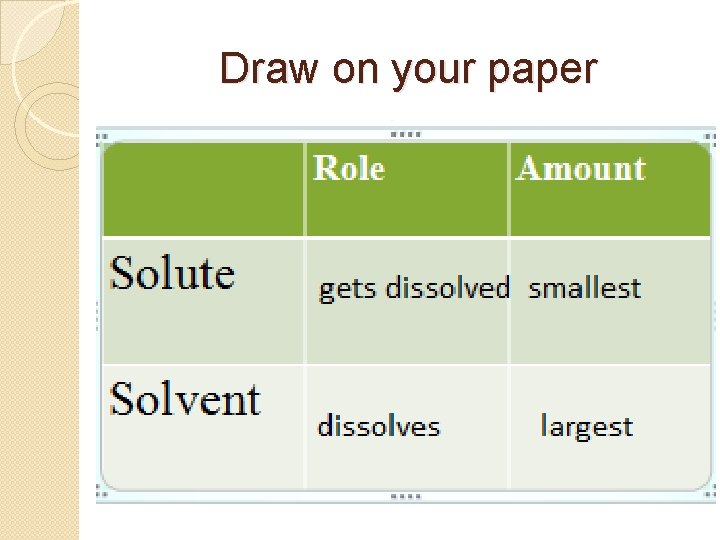
Draw on your paper Role Solute Solvent Amount

Use these words to fill in your chart Gets dissolved Dissolves Smallest Largest
Draw on your paper

Circle the solute, underline the solvent �Solution of 20% HCl, 80% water �Solution of 55% HCl, 45% water �Isopropyl alcohol: 91% alcohol, 9% water �Rubbing alcohol: 60% alcohol, 40% water �Salt water �Sugar water �Chocolate milk
Circle the solute, underline the solvent Remember �Solute (smaller word) – least amount �Solvent (longer word) – larger amount

Circle the solute, underline the solvent �Solution of 20% HCl, 80% water �Solution of 55% HCl, 45% water �Isopropyl alcohol: 91% alcohol, 9% water �Rubbing alcohol: 60% alcohol, 40% water �Salt water �Sugar water �Chocolate milk

Circle the solute, underline the solvent
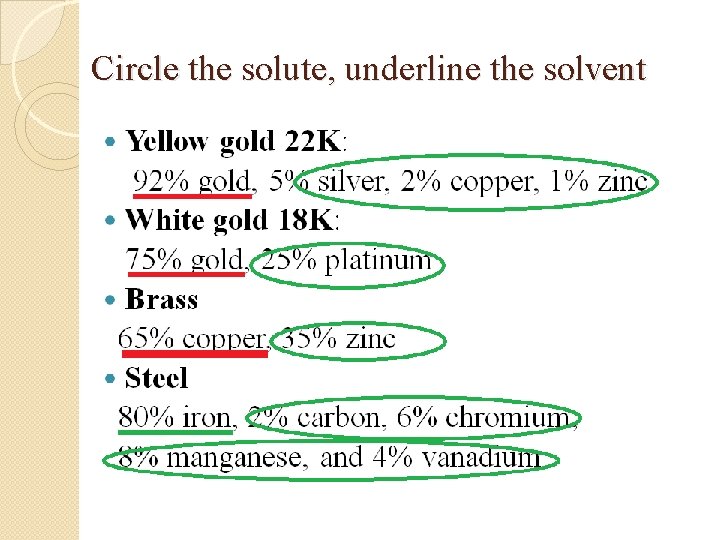
Circle the solute, underline the solvent �Yellow gold 22 K: 92% gold, 5% silver, 2% copper, 1% zinc �White gold 18 K: 75% gold, 25% platinum �Brass 65% copper, 35% zinc �Steel 80% iron, 2% carbon, 6% chromium, 8% manganese, and 4% vanadium
Circle the solute, underline the solvent
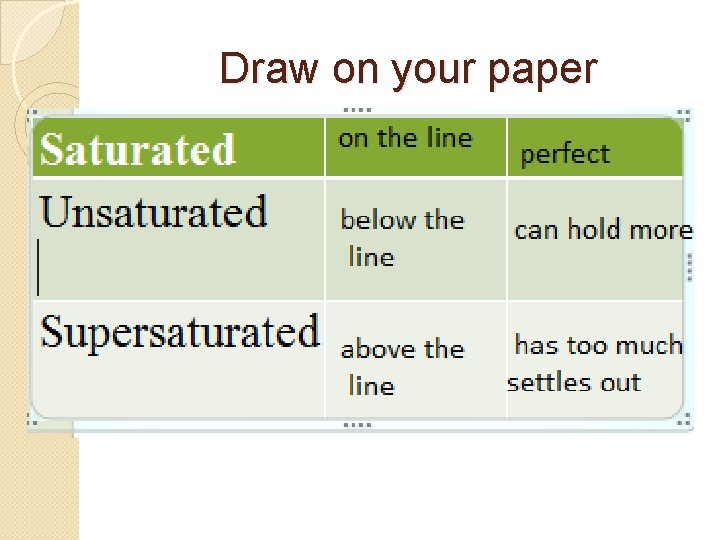
Draw on your paper Saturated Unsaturated Supersaturated

Use these words to fill in your chart Square 1: Above the line Below the line On the line Square 2: Can hold more solute Holds perfect amount of solute Has too much solute, some settles out
Draw on your paper
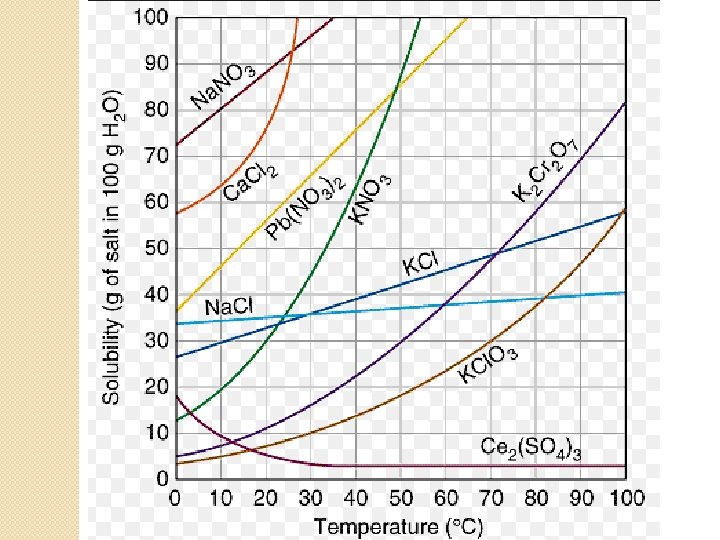
Graph Questions �Which solution decreases in solubility as temperature increases? �Which solution is the least effected by an increase in temperature?
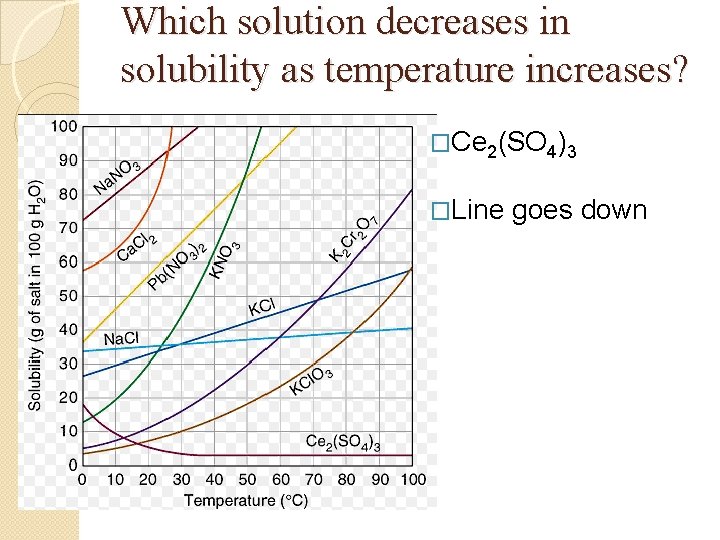
Which solution decreases in solubility as temperature increases? �Ce 2(SO 4)3 �Line goes down
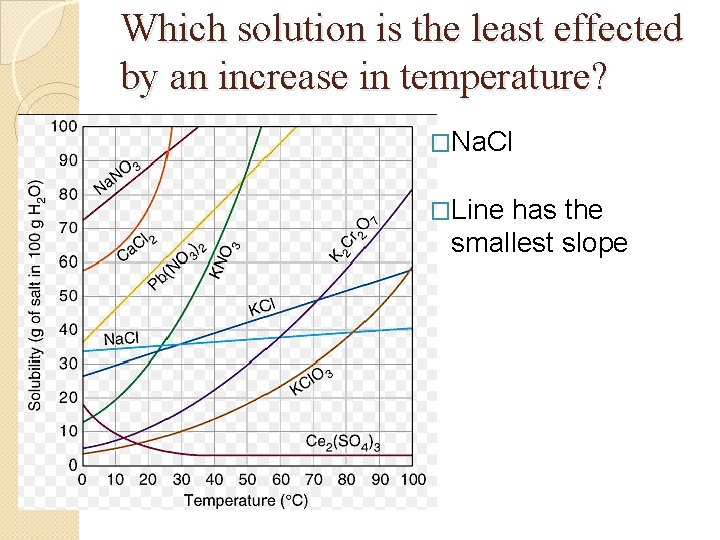
Which solution is the least effected by an increase in temperature? �Na. Cl �Line has the smallest slope
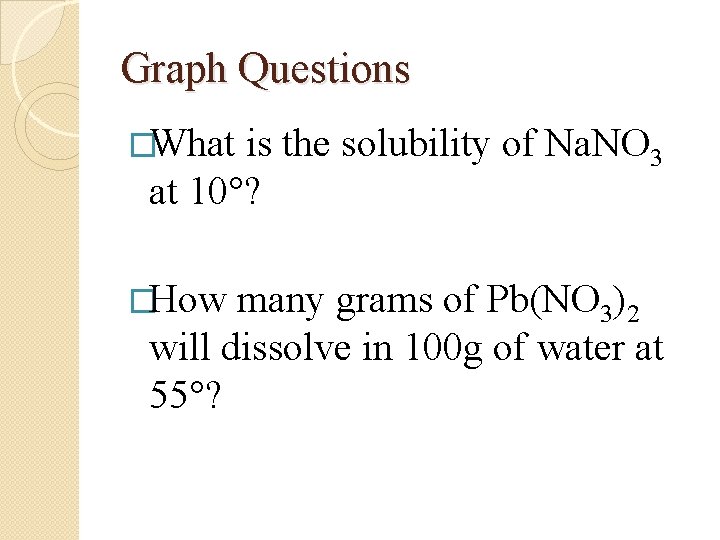
Graph Questions �What is the solubility of Na. NO 3 at 10°? �How many grams of Pb(NO 3)2 will dissolve in 100 g of water at 55°?
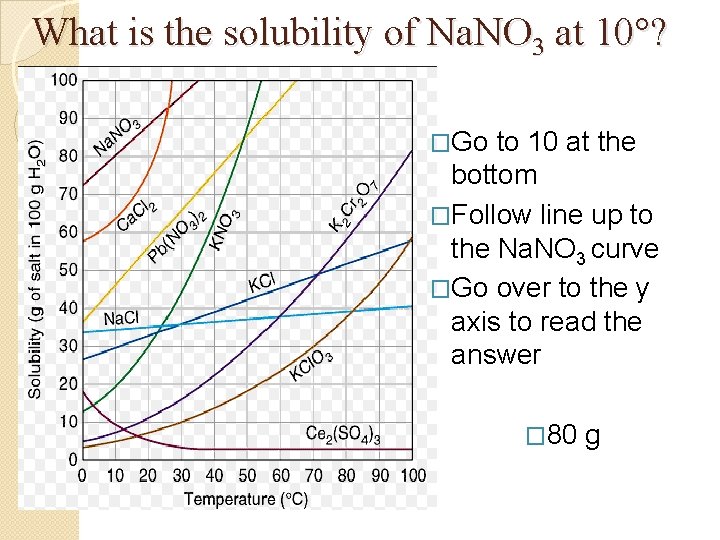
What is the solubility of Na. NO 3 at 10°? �Go to 10 at the bottom �Follow line up to the Na. NO 3 curve �Go over to the y axis to read the answer � 80 g
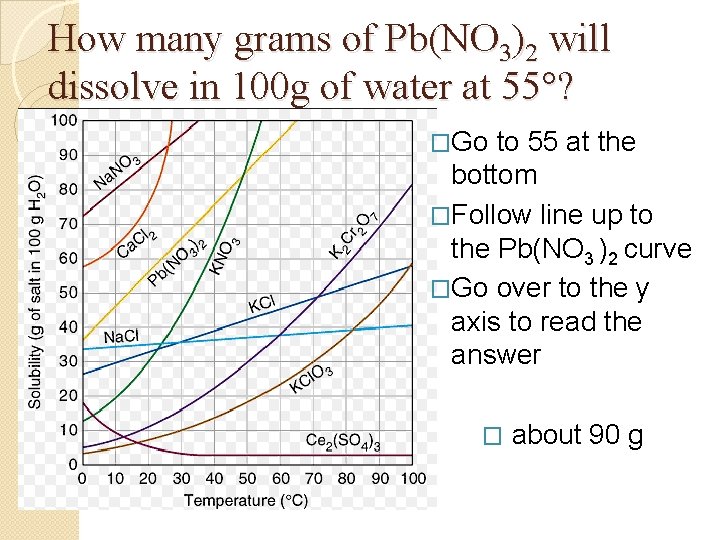
How many grams of Pb(NO 3)2 will dissolve in 100 g of water at 55°? �Go to 55 at the bottom �Follow line up to the Pb(NO 3 )2 curve �Go over to the y axis to read the answer � about 90 g
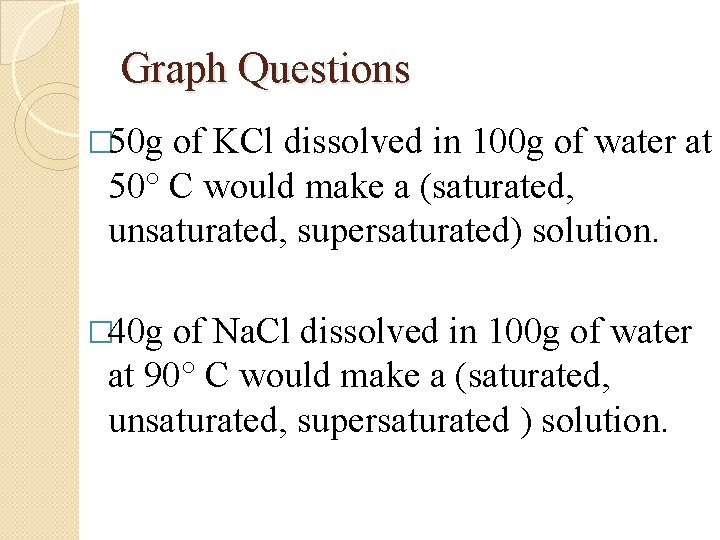
Graph Questions � 50 g of KCl dissolved in 100 g of water at 50° C would make a (saturated, unsaturated, supersaturated) solution. � 40 g of Na. Cl dissolved in 100 g of water at 90° C would make a (saturated, unsaturated, supersaturated ) solution.
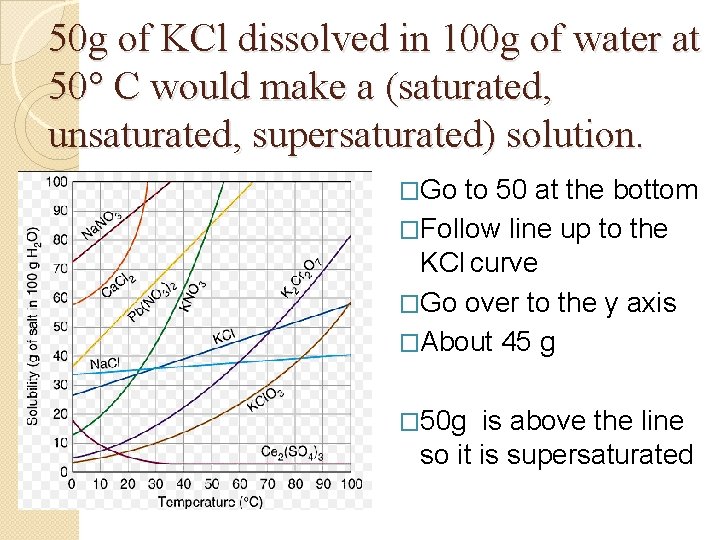
50 g of KCl dissolved in 100 g of water at 50° C would make a (saturated, unsaturated, supersaturated) solution. �Go to 50 at the bottom �Follow line up to the KCl curve �Go over to the y axis �About 45 g � 50 g is above the line so it is supersaturated
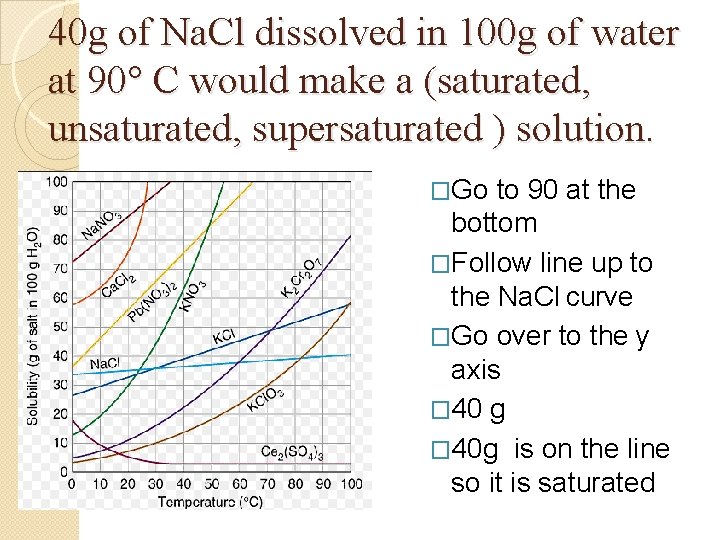
40 g of Na. Cl dissolved in 100 g of water at 90° C would make a (saturated, unsaturated, supersaturated ) solution. �Go to 90 at the bottom �Follow line up to the Na. Cl curve �Go over to the y axis � 40 g � 40 g is on the line so it is saturated

Graph Questions � 50 g of Ca. Cl 2 dissolved in 100 g of water at 10° C would make a (saturated, unsaturated, supersaturated ) solution. � 20 g of K 2 Cr 2 O 7 dissolved in 100 g of water at 20° C would make a (saturated, unsaturated, supersaturated) solution.
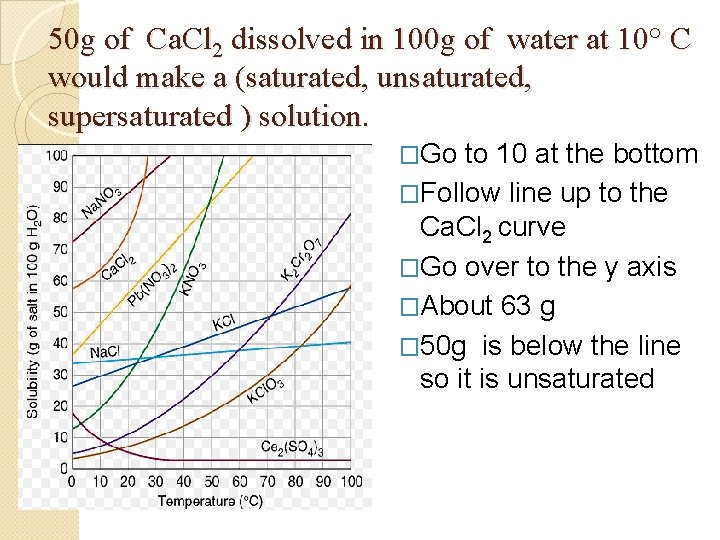
50 g of Ca. Cl 2 dissolved in 100 g of water at 10° C would make a (saturated, unsaturated, supersaturated ) solution. . �Go to 10 at the bottom �Follow line up to the Ca. Cl 2 curve �Go over to the y axis �About 63 g � 50 g is below the line so it is unsaturated
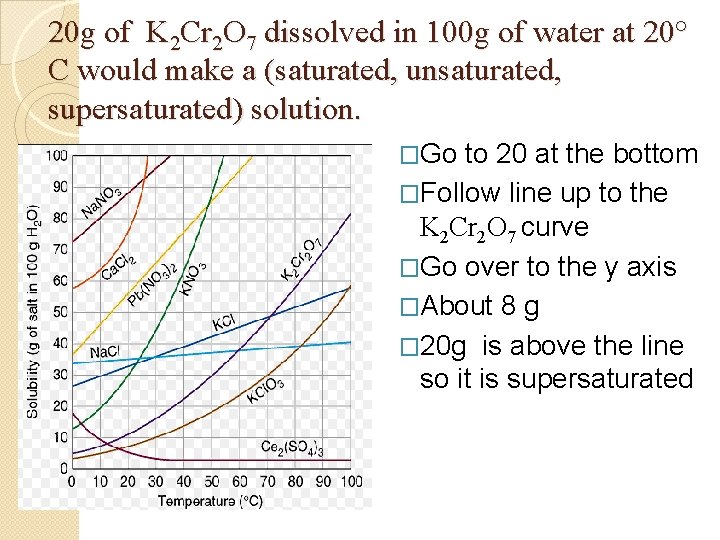
20 g of K 2 Cr 2 O 7 dissolved in 100 g of water at 20° C would make a (saturated, unsaturated, supersaturated) solution. . �Go to 20 at the bottom . �Follow line up to the K 2 Cr 2 O 7 curve �Go over to the y axis �About 8 g � 20 g is above the line so it is supersaturated
- Slides: 40