Solutions Review Chemistry Which of the following operation


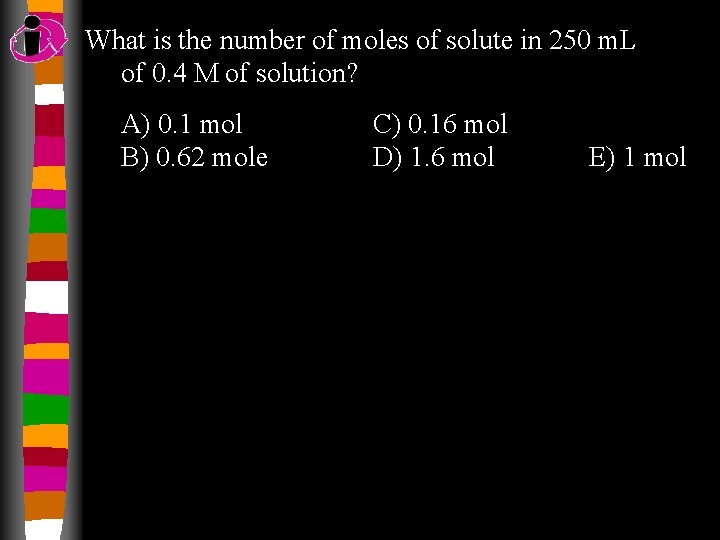





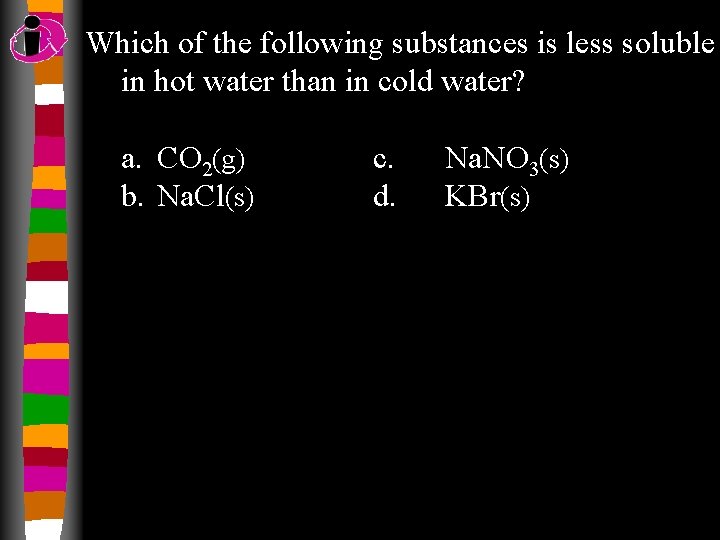



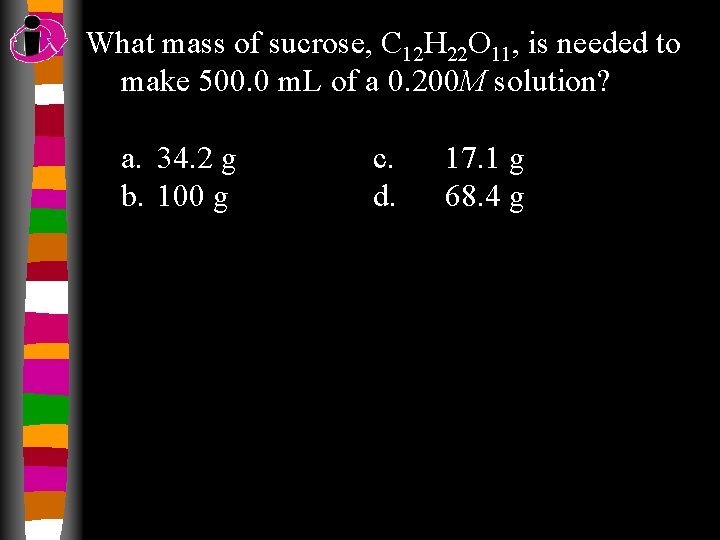

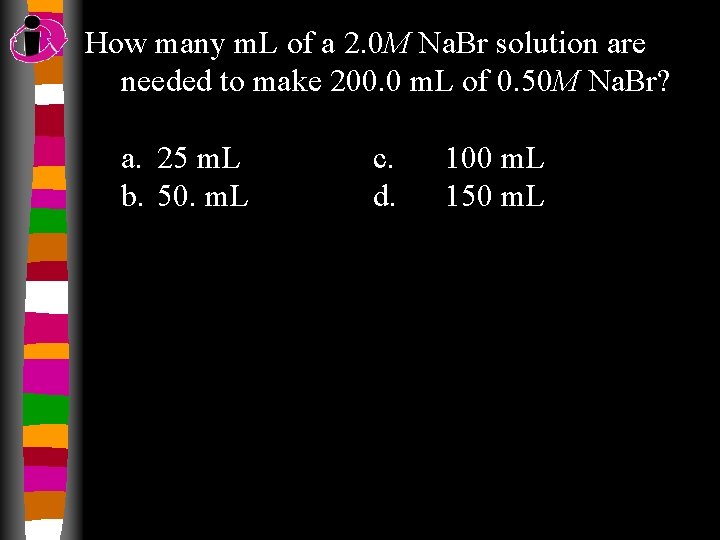

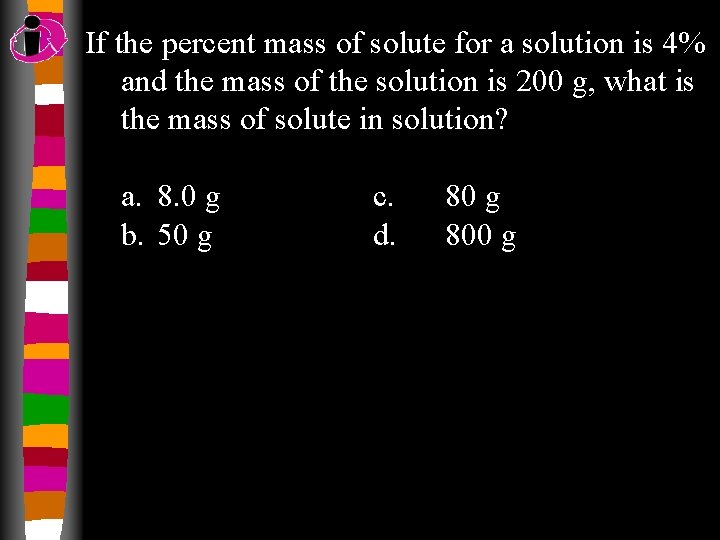








- Slides: 27

Solutions Review Chemistry

Which of the following operation usually makes a substance dissolve faster in a solvent? A) agitation B) raising the temperature C) crushing the substance into a powder D) all of the above.

Which of the following is a colligative property that explains why ice will form at higher temperatures in the Great Lakes than in the ocean. a. b. c. d. e. boiling point elevation molarity molality freezing point depression mole fraction
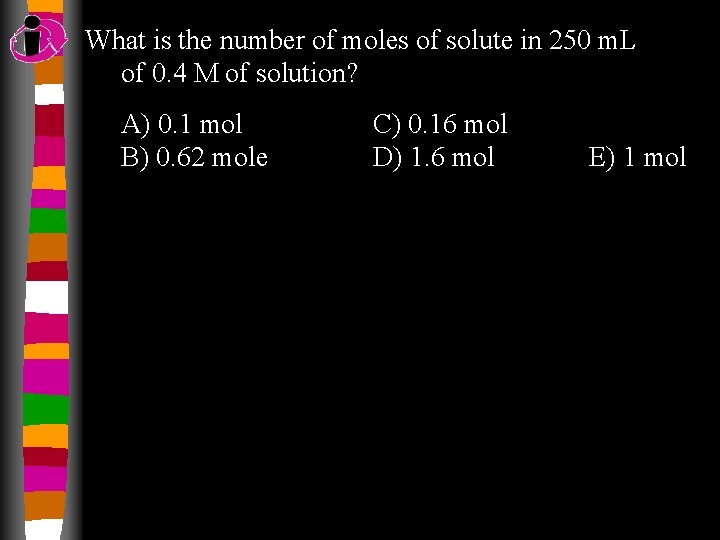
What is the number of moles of solute in 250 m. L of 0. 4 M of solution? A) 0. 1 mol B) 0. 62 mole C) 0. 16 mol D) 1. 6 mol E) 1 mol

Pressure has and influence on the solubility of: A) liquids dissolving in liquids B) solids & liquids dissolving in liquids. C) gases dissolving in liquids. D) solids dissolving in liquids

Colligative properties of solutions depend on: A) size of the solute particles. B) molecular nature of the solute particles C) charge of the solute particles. D) the number of solute particles

What is the maximum amount of KCl that can dissolve in 200 g of water? (The solubility of KCl is 34 g/100 g H 2 O at 20 C. ) a. 17 g b. 34 g c. d. 68 g 6800 g

Which of the following pairs of factors affects the solubility of a particular substance? a. b. c. d. particle size and degree of mixing temperature and the nature of solute and solvent particle size and temperature

If a crystal added to an aqueous solution causes many particles to come out of the solution, the original solution was ____. a. unsaturated b. saturated c. an emulsion d. supersaturated
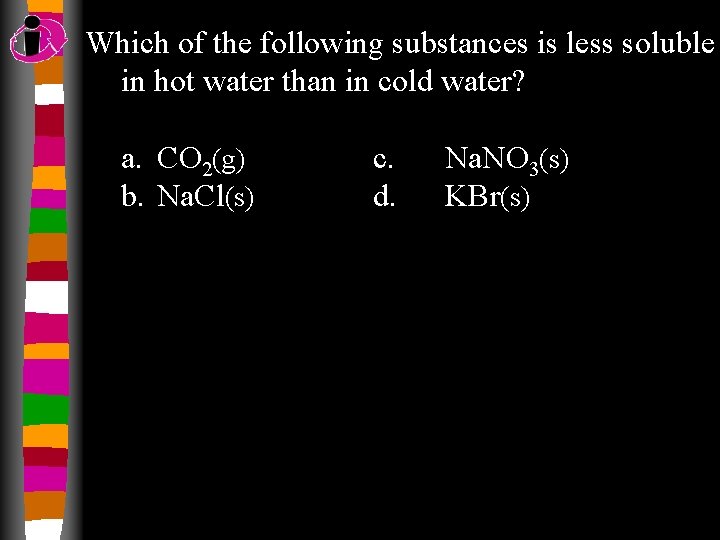
Which of the following substances is less soluble in hot water than in cold water? a. CO 2(g) b. Na. Cl(s) c. d. Na. NO 3(s) KBr(s)

Which of the following occurs to most solids as temperature increases? a. Solubility decreases. b. Solubility increases. c. Solubility remains the same. d. Molarity doubles.

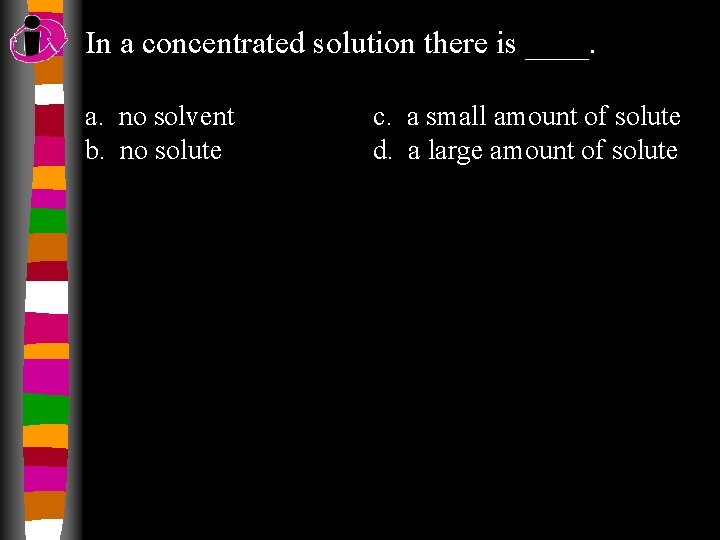
In a concentrated solution there is ____. a. no solvent b. no solute c. a small amount of solute d. a large amount of solute

What is the molarity of a solution that contains 6 moles of solute in 2 liters of solution? a. 6 M c. 7 M b. 12 M d. 3 M

Which of the following operations yields the number of moles of solute? a. molarity x moles of solution b. molarity x mass of solution c. molarity x liters of solution d. moles of solution x volume of solution
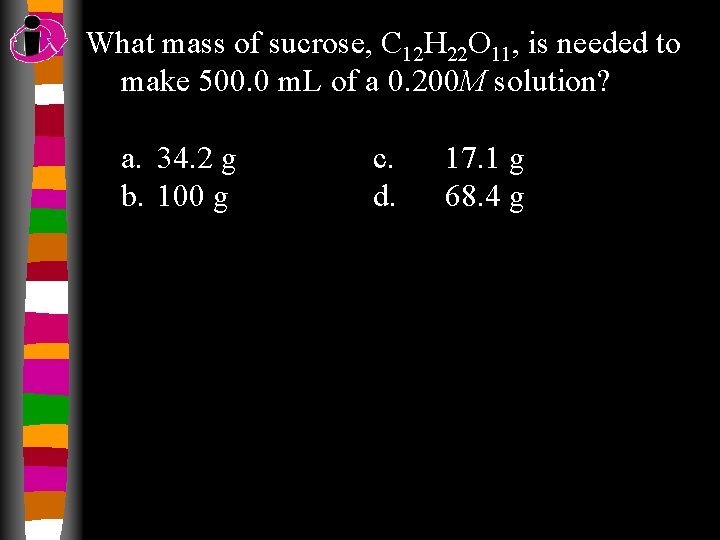
What mass of sucrose, C 12 H 22 O 11, is needed to make 500. 0 m. L of a 0. 200 M solution? a. 34. 2 g b. 100 g c. d. 17. 1 g 68. 4 g

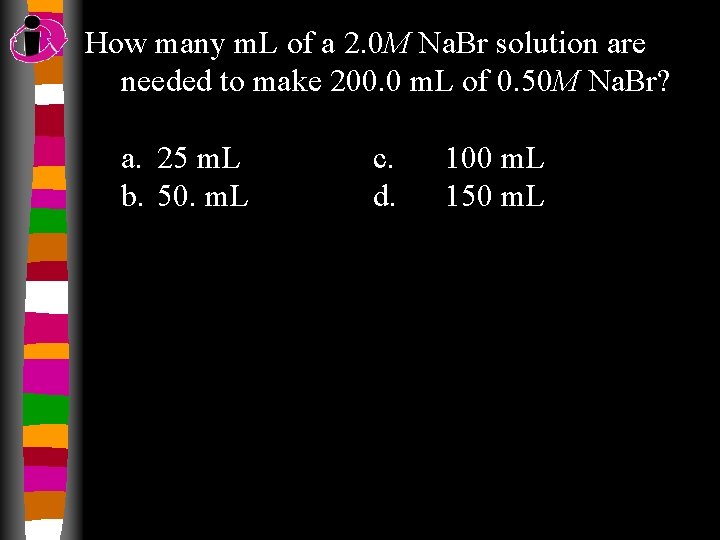
How many m. L of a 2. 0 M Na. Br solution are needed to make 200. 0 m. L of 0. 50 M Na. Br? a. 25 m. L b. 50. m. L c. d. 100 m. L 150 m. L

If 2. 0 m. L of 6. 0 M HCl is used to make a 500. 0 m. L aqueous solution, what is the molarity of the dilute solution? a. 0. 024 M b. 0. 24 M c. d. 0. 30 M 0. 83 M
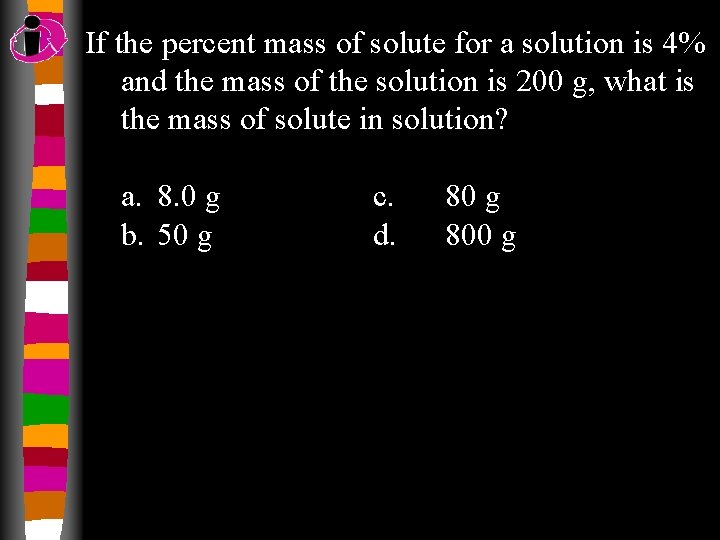
If the percent mass of solute for a solution is 4% and the mass of the solution is 200 g, what is the mass of solute in solution? a. 8. 0 g b. 50 g c. d. 80 g 800 g

Which of the following is NOT a colligative property of a solution? a. b. c. d. boiling point elevation vapor pressure lowering supersaturation freezing point depression

What is the mole fraction of ethanol in a solution of 3. 00 moles of ethanol and 5. 00 moles of water? a. 0. 375 b. 0. 6 c. d. 1. 67 15

What is the molality of a solution containing 8. 0 grams of solute in 0. 50 kg of solvent? (molar mass of solute = 24 g) a. 0. 67 m b. 4. 0 m c. d. 1. 7 m 0. 17 m

What is the number of kilograms of solvent in a 0. 70 molal solution containing 5. 0 grams of solute? (molar mass of solute = 30 g) a. 0. 24 kg b. 2. 4 kg c. d. 0. 11 kg 1. 1 kg

To which of the following variables is change in boiling point directly proportional? a. b. c. d. molarity of solution percent by volume of solution molality of solution percent (mass/mass) of solution

The freezing point of a solution that contains 0. 550 moles of Na. I in 615 g of water is ____. (kf = 1. 86 C/m; molar mass of water = 18. 0 g) a. 1. 66 C b. -1. 66 C c. d. 3. 33 C -3. 33 C

What is the boiling point of a solution that contains 3. 00 moles of KBr in 2000. m. L of water? (kb = 0. 512 C/m; molar mass of water = 18. 0 g) a. 97. 0 C b. 99. 7 C c. d. 101. 4 C 103. 0 C

What is the approximate molar mass of a molecular solute if 300. g of the solute in 1000. g of water causes the solution to have a boiling point of 101 C? (kb = 0. 512 C/m; kf = 1. 86 C/m; molar mass of water = 18. 0 g) a. 15. 0 g/mol b. 30. 0 g/mol c. d. 150. g/mol 300. g/mol
 Which of the following suffixes refers to eating?
Which of the following suffixes refers to eating? Carriage of a lathe consists of__________.
Carriage of a lathe consists of__________. Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Regents
Regents Chapter 12 review solutions section 2
Chapter 12 review solutions section 2 Chapter 13 mixtures and solutions answers
Chapter 13 mixtures and solutions answers Modern chemistry solutions
Modern chemistry solutions Ap chemistry solutions
Ap chemistry solutions Living by chemistry solutions
Living by chemistry solutions A slow jogger runs a mile in 13 minutes
A slow jogger runs a mile in 13 minutes Metric dimensional analysis worksheet
Metric dimensional analysis worksheet What is catalystfive
What is catalystfive Chemistry in biology section 3 water and solutions
Chemistry in biology section 3 water and solutions Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry 7-1 practice problems chemistry answers
7-1 practice problems chemistry answers Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Grade 10 chemistry review with answers
Grade 10 chemistry review with answers Chemistry sol review packet
Chemistry sol review packet Chemistry unit review answer key
Chemistry unit review answer key Kuhinjska sol formula
Kuhinjska sol formula Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 What functional group is ch3
What functional group is ch3 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Ap chemistry equilibrium review
Ap chemistry equilibrium review Ap chemistry big idea 2 review answers
Ap chemistry big idea 2 review answers