Mixtures and Solutions Solute and Solvent Matter Heterogeneous
















- Slides: 51

Mixtures and Solutions Solute and Solvent

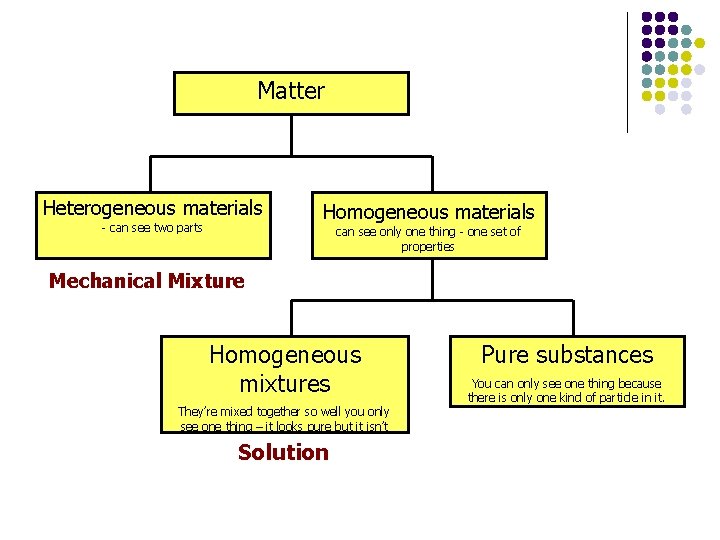
Matter Heterogeneous materials - can see two parts Homogeneous materials can see only one thing - one set of properties Mechanical Mixture Homogeneous mixtures They’re mixed together so well you only see one thing – it looks pure but it isn’t Solution Pure substances You can only see one thing because there is only one kind of particle in it.

Review Solutions l Are solutions homogeneous or heterogeneous? l l Are solutions mixtures or pure substances? l l Mixtures What kind of states can a solution be? l l Homogeneous Solid, liquid, or gas What are the two “s” words that every solution must have? l A solute and a solvent

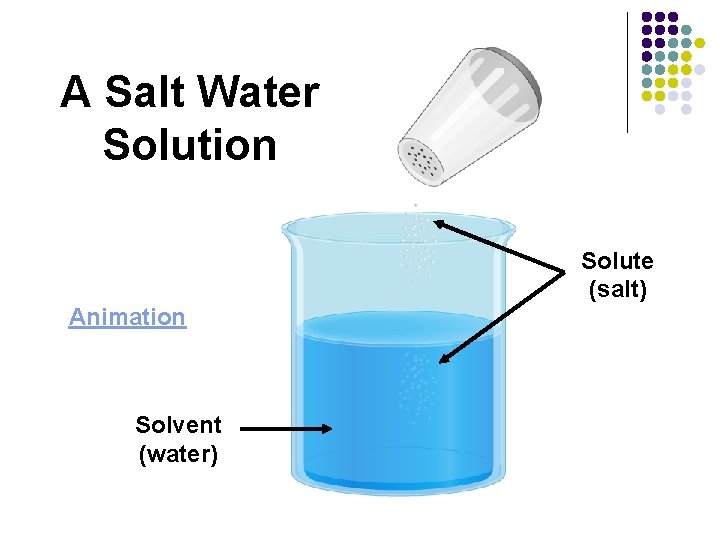
In a salt water solution… l Is salt the solute or the solvent? l l Is water the solute or the solvent? l l Solvent What does the solute do? l l Solute Gets dissolved What does the solvent do? l Does the dissolving

A Salt Water Solution Solute (salt) Animation Solvent (water)

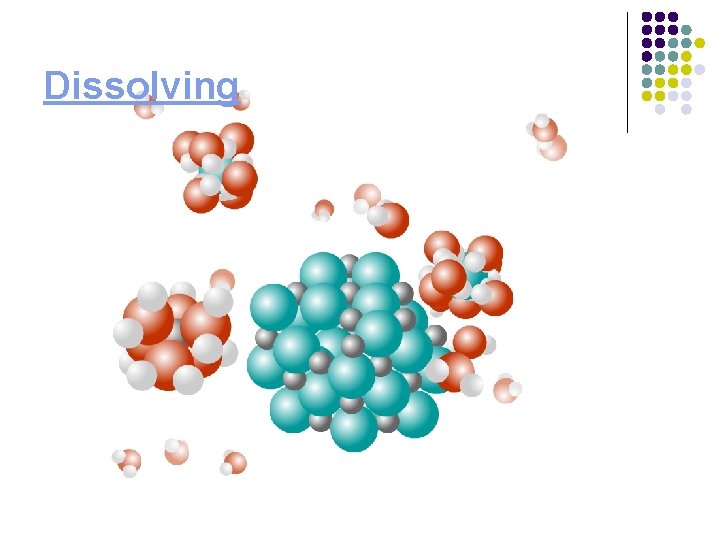
Dissolving

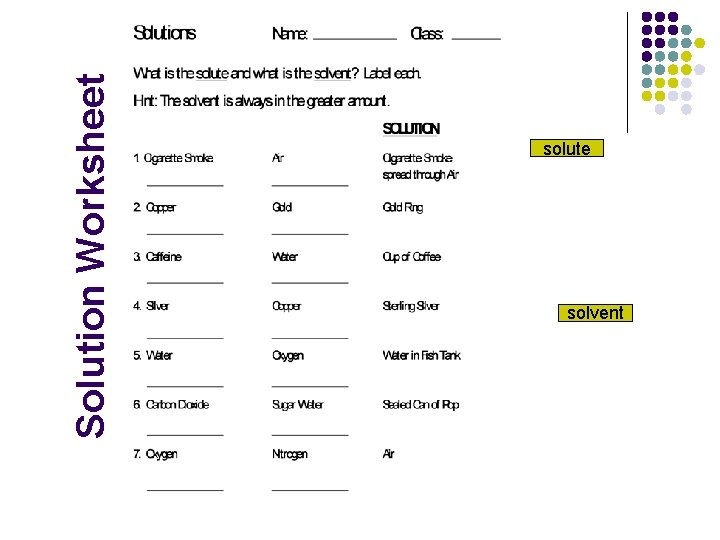
Solution Worksheet solute solvent

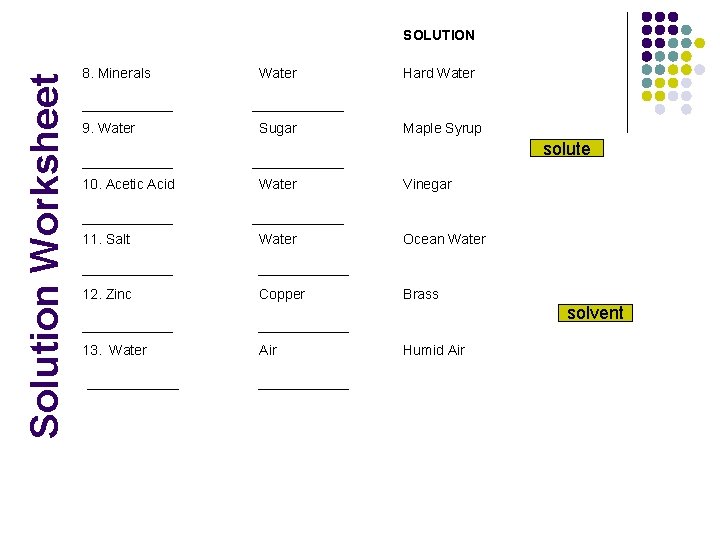
Solution Worksheet SOLUTION 8. Minerals Water Hard Water 9. Water Sugar Maple Syrup solute 10. Acetic Acid Water Vinegar 11. Salt Water Ocean Water 12. Zinc Copper Brass solvent 13. Water Air Humid Air

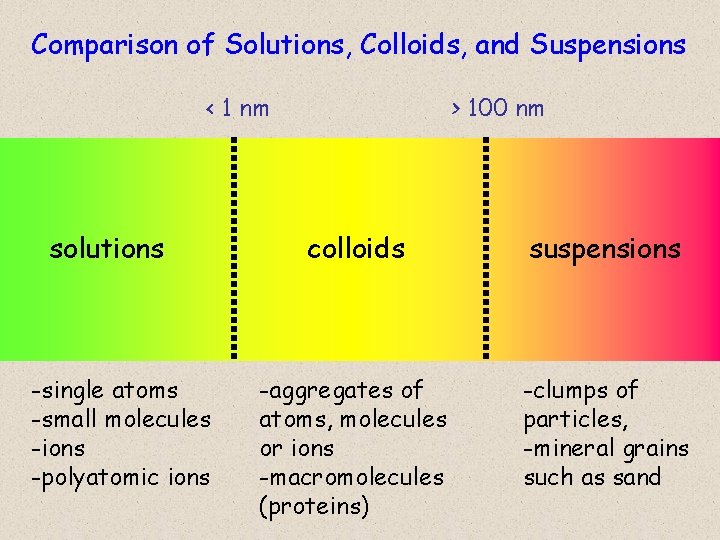
Comparison of Solutions, Colloids, and Suspensions < 1 nm solutions -single atoms -small molecules -ions -polyatomic ions > 100 nm colloids suspensions -aggregates of atoms, molecules or ions -macromolecules (proteins) -clumps of particles, -mineral grains such as sand

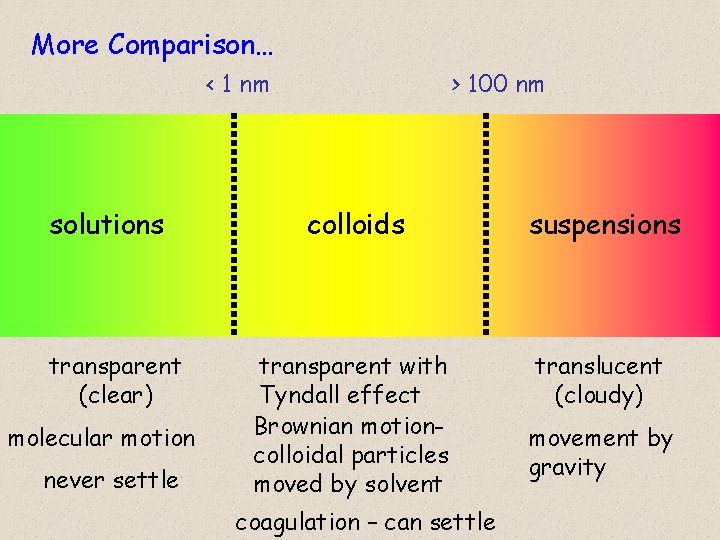
More Comparison… < 1 nm solutions transparent (clear) molecular motion never settle > 100 nm colloids suspensions transparent with Tyndall effect Brownian motioncolloidal particles moved by solvent translucent (cloudy) coagulation – can settle movement by gravity

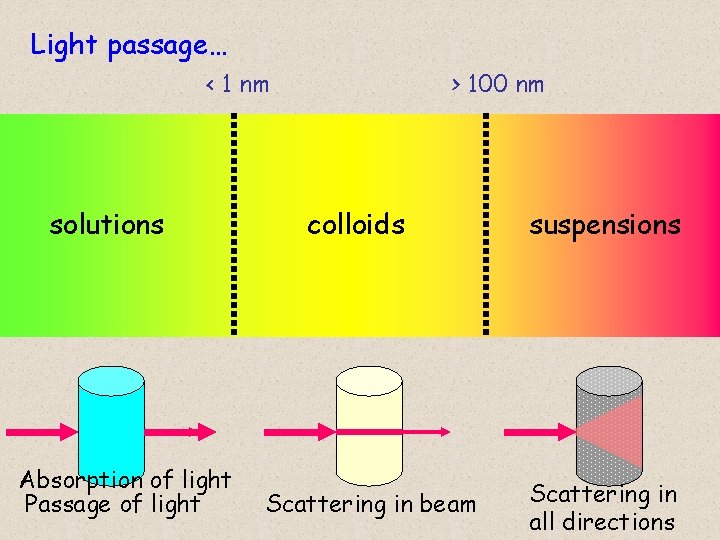
Light passage… < 1 nm solutions Absorption of light Passage of light > 100 nm colloids Scattering in beam suspensions Scattering in all directions

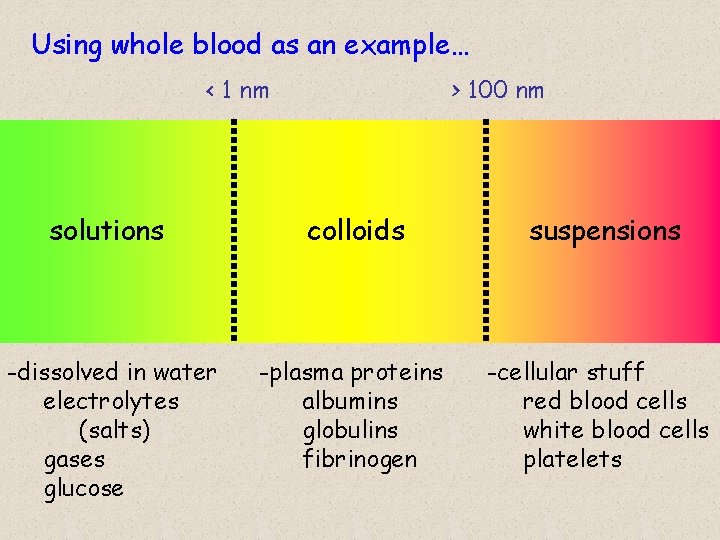
Using whole blood as an example… < 1 nm > 100 nm solutions colloids suspensions -dissolved in water electrolytes (salts) gases glucose -plasma proteins albumins globulins fibrinogen -cellular stuff red blood cells white blood cells platelets

Solutions: homogeneous mixtures • Absence of settling • Two components (at the least)– Solute – the substance being dissolved – Solvent – the dissolving medium • usually water – aqueous solution can have multi-solute solutions - seawater

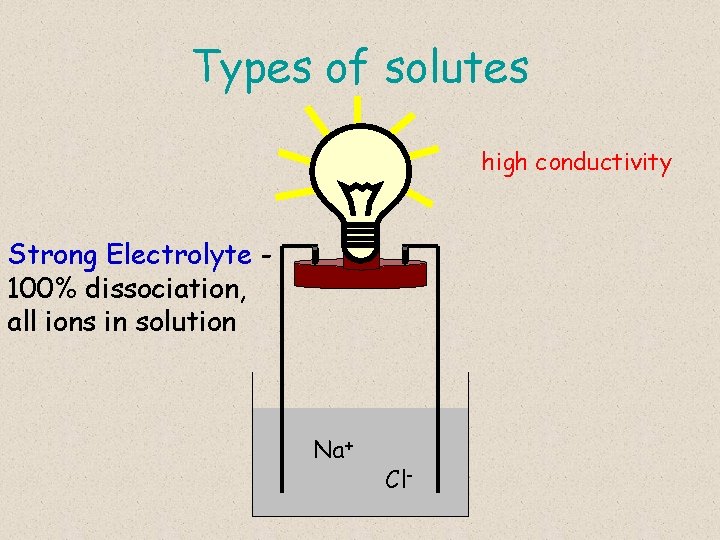
Types of solutes high conductivity Strong Electrolyte 100% dissociation, all ions in solution Na+ Cl-

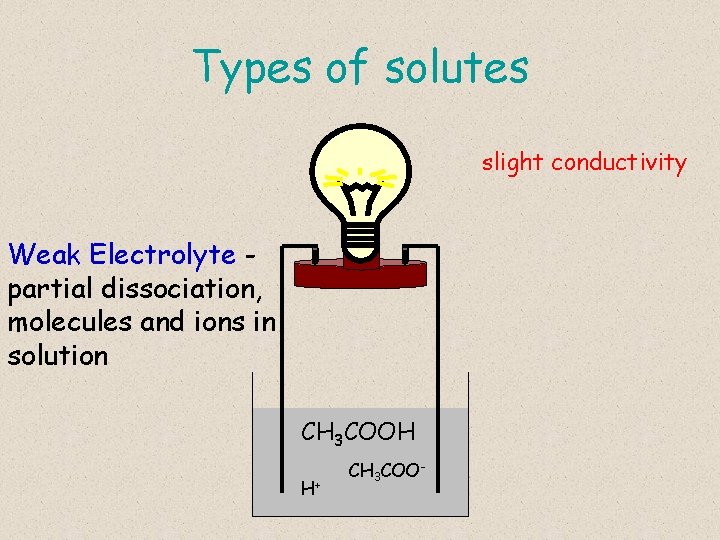
Types of solutes slight conductivity Weak Electrolyte partial dissociation, molecules and ions in solution CH 3 COOH H+ CH 3 COO-

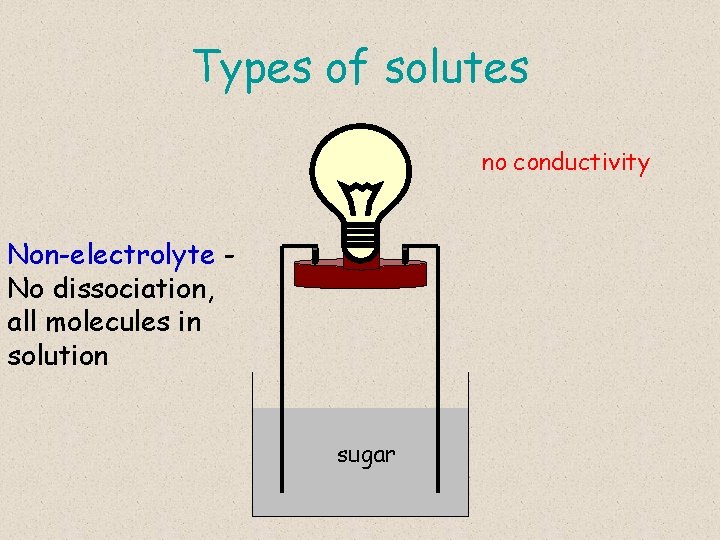
Types of solutes no conductivity Non-electrolyte No dissociation, all molecules in solution sugar

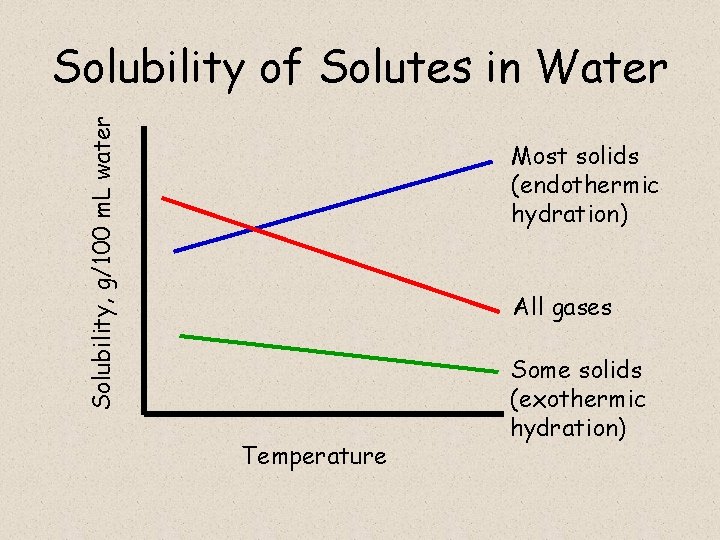
Solubility, g/100 m. L water Solubility of Solutes in Water Most solids (endothermic hydration) All gases Temperature Some solids (exothermic hydration)

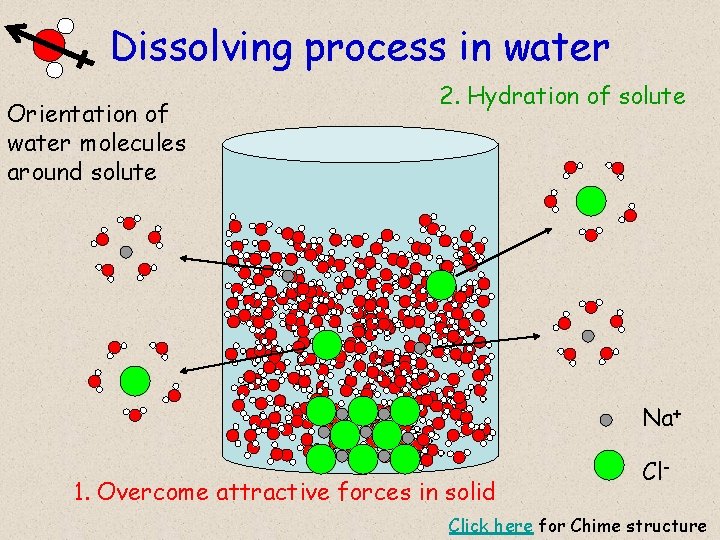
Dissolving process in water Orientation of water molecules around solute 2. Hydration of solute Na+ 1. Overcome attractive forces in solid Cl- Click here for Chime structure

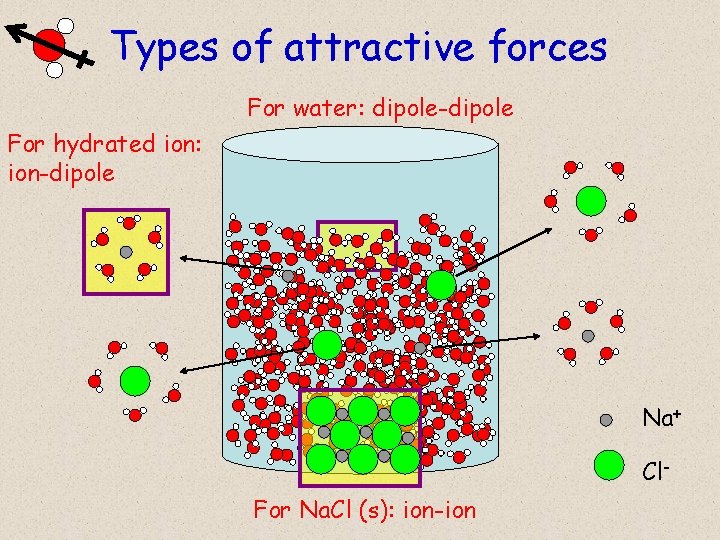
Types of attractive forces For water: dipole-dipole For hydrated ion: ion-dipole Na+ Cl. For Na. Cl (s): ion-ion

How do I get sugar to dissolve faster in my iced tea? Stir, and stir Fresh solvent contact and interaction with solute Add sugar to warm tea then add ice Faster rate of dissolution at higher temperature Grind the sugar to a powder Greater surface area, more solute-solvent interaction

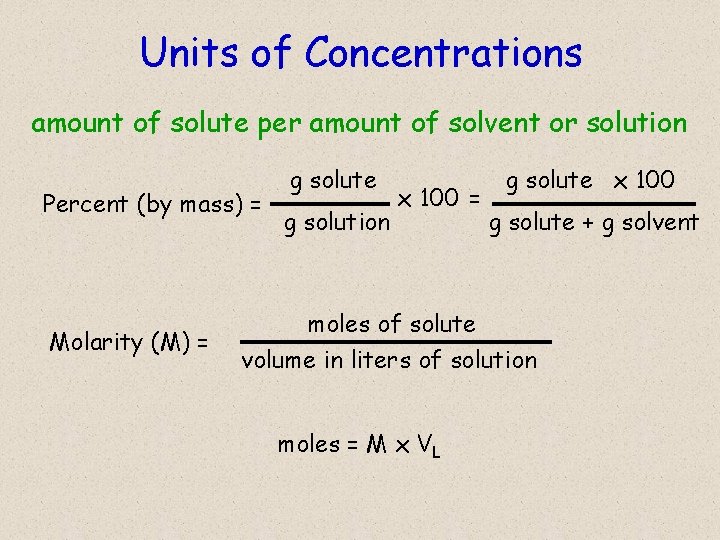
Units of Concentrations amount of solute per amount of solvent or solution Percent (by mass) = Molarity (M) = g solute g solution x 100 = g solute x 100 g solute + g solvent moles of solute volume in liters of solution moles = M x VL

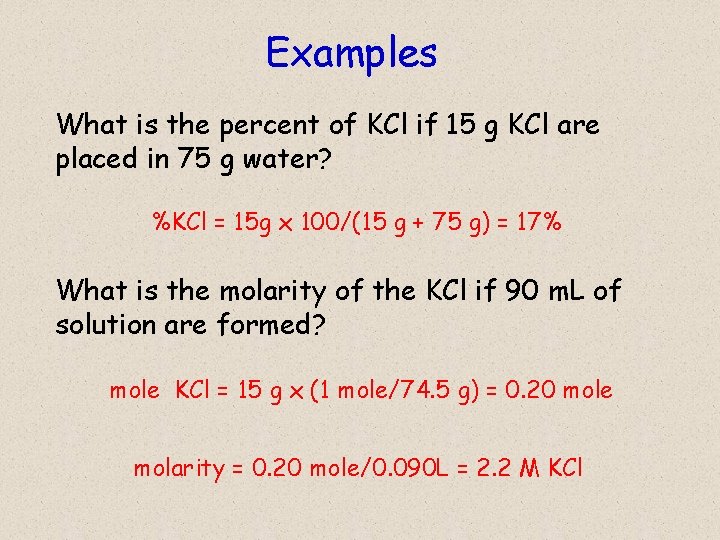
Examples What is the percent of KCl if 15 g KCl are placed in 75 g water? %KCl = 15 g x 100/(15 g + 75 g) = 17% What is the molarity of the KCl if 90 m. L of solution are formed? mole KCl = 15 g x (1 mole/74. 5 g) = 0. 20 mole molarity = 0. 20 mole/0. 090 L = 2. 2 M KCl

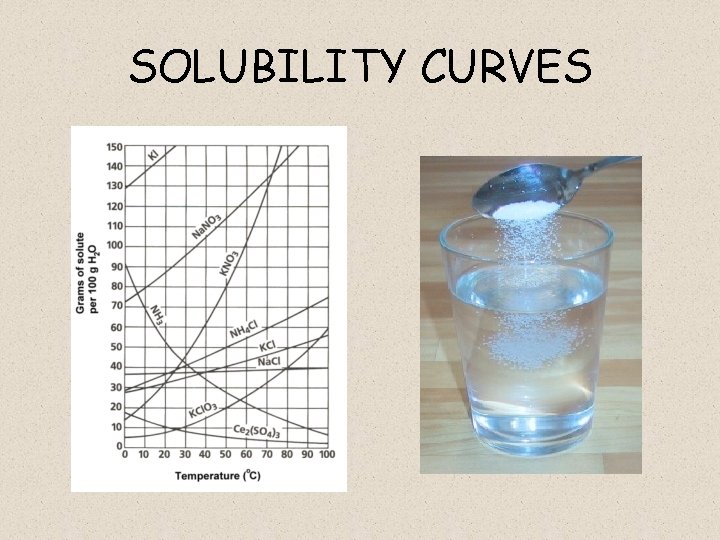
SOLUBILITY CURVES

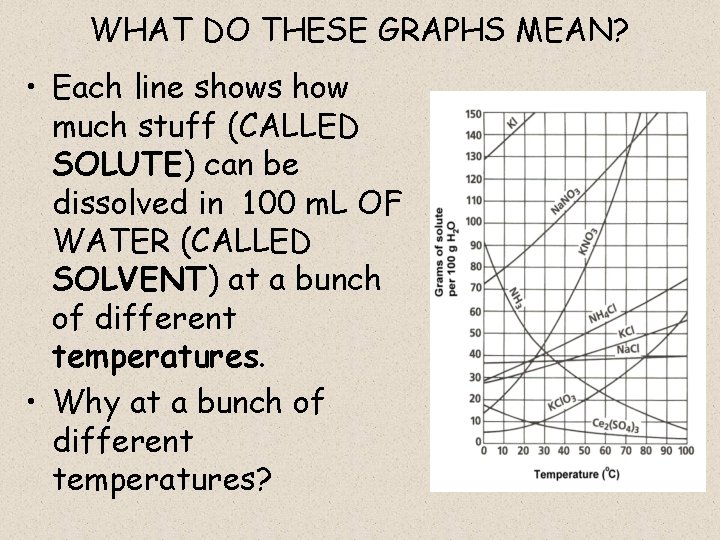
WHAT DO THESE GRAPHS MEAN? • Each line shows how much stuff (CALLED SOLUTE) can be dissolved in 100 m. L OF WATER (CALLED SOLVENT) at a bunch of different temperatures. • Why at a bunch of different temperatures?

WHAT DOES THE LINES REPRESENT? • They represents SATURATED SOLUTIONS at a given temperature: – SATURATED = cannot dissolve any more solute

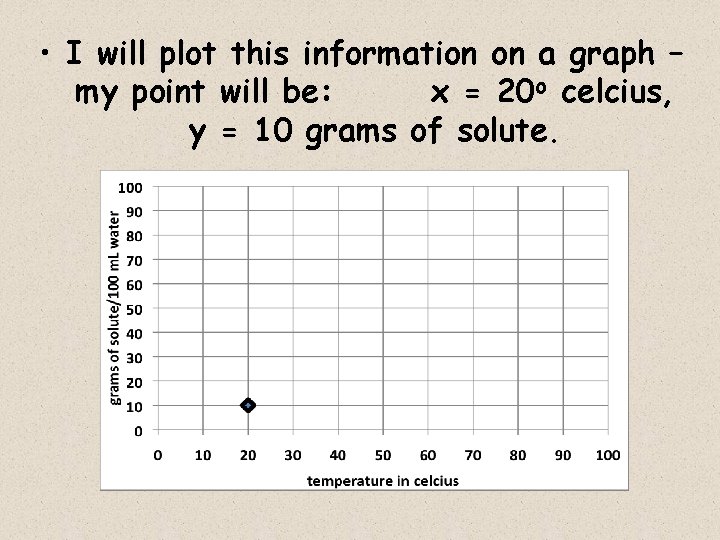
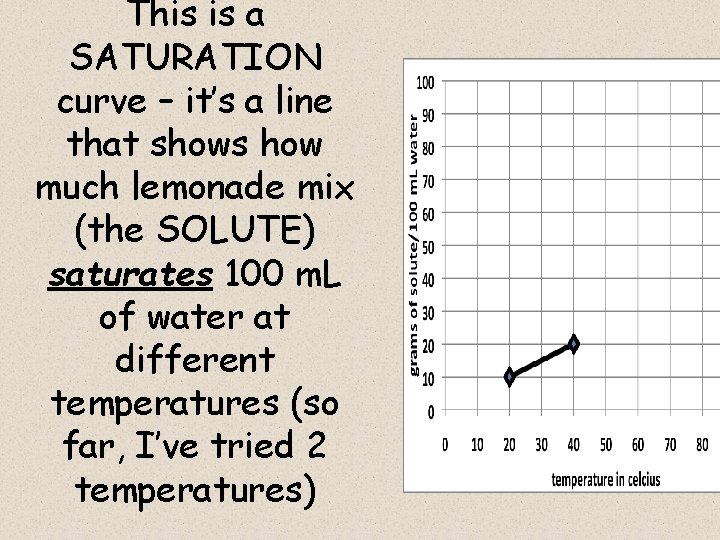
WHAT DO THE LINES REPRESENT? continued For example, let’s say I put a 10 grams of powdered lemonade mix into a 100 ml glass of water, and stir until dissolved I then try to add 1 more gram, but it won’t dissolve – it just piles up on the bottom of the glass. • This shows me that at a water temperature of 20 o celcius, 100 m. L of water is saturated by 10 grams of lemonade mix – it cannot dissolve 11 grams (or more) of mix

• I will plot this information on a graph – my point will be: x = 20 o celcius, y = 10 grams of solute.

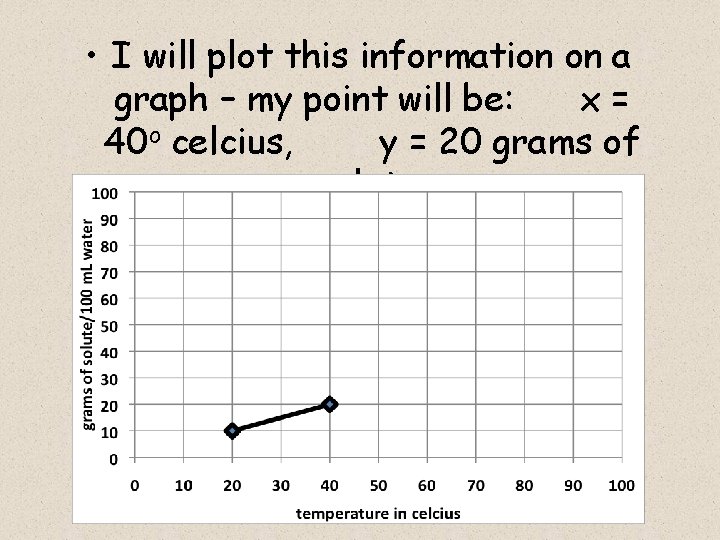
Next, I heated 100 m. L of water up to 40 o celcius, and I added 20 grams of powdered lemonade mix into the heated glass of water, and I stirred until it all dissolved I then try to add 1 more gram, but it won’t dissolve – it just piles up on the bottom of the glass.

This shows me that at a water temperature of 40 o celcius, 100 m. L of water is SATURATED by 20 grams of lemonade mix – it cannot dissolve 21 grams (or more) of mix

• I will plot this information on a graph – my point will be: x= 40 o celcius, y = 20 grams of solute.

This is a SATURATION curve – it’s a line that shows how much lemonade mix (the SOLUTE) saturates 100 m. L of water at different temperatures (so far, I’ve tried 2 temperatures)

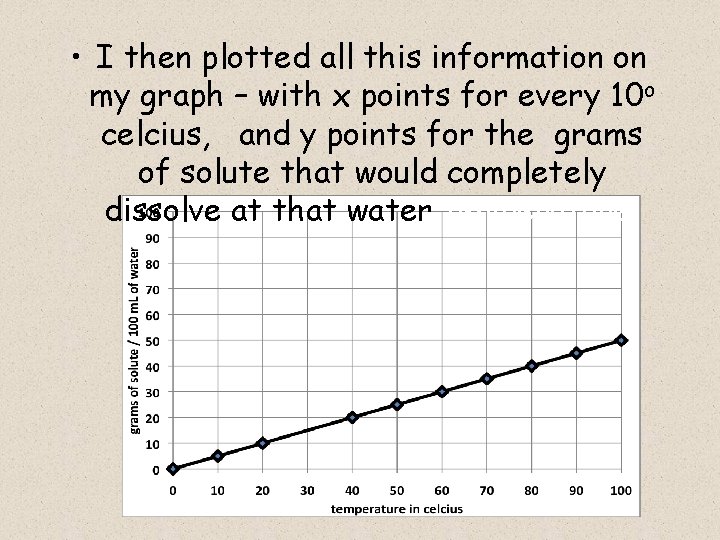
Next, I continued to heat 100 m. L of water up by 10 o celcius, and I kept adding grams of powdered lemonade mix into the heated glass of water, and I stirred until it all dissolved.

For each new temperature, I would then try to add 1 more gram, but it won’t dissolve – it just piles up on the bottom of the glass. At that point, I’d heat up the water again, and repeat the

• I then plotted all this information on my graph – with x points for every 10 o celcius, and y points for the grams of solute that would completely dissolve at that water temperature:

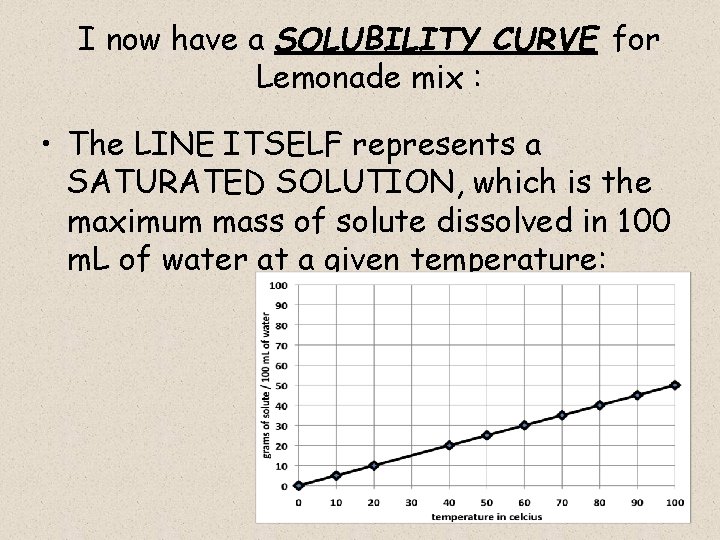
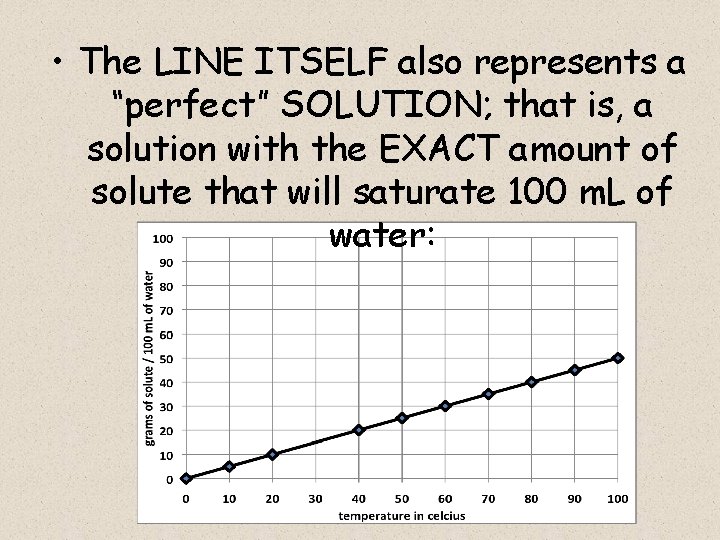
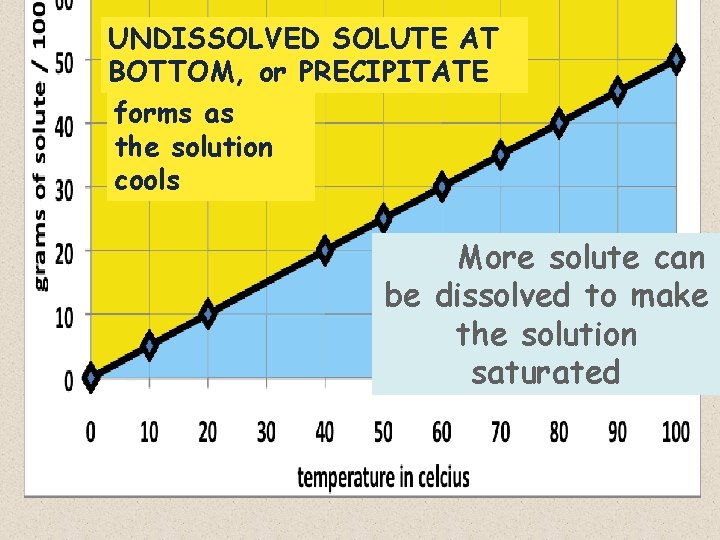
I now have a SOLUBILITY CURVE for Lemonade mix : • The LINE ITSELF represents a SATURATED SOLUTION, which is the maximum mass of solute dissolved in 100 m. L of water at a given temperature:

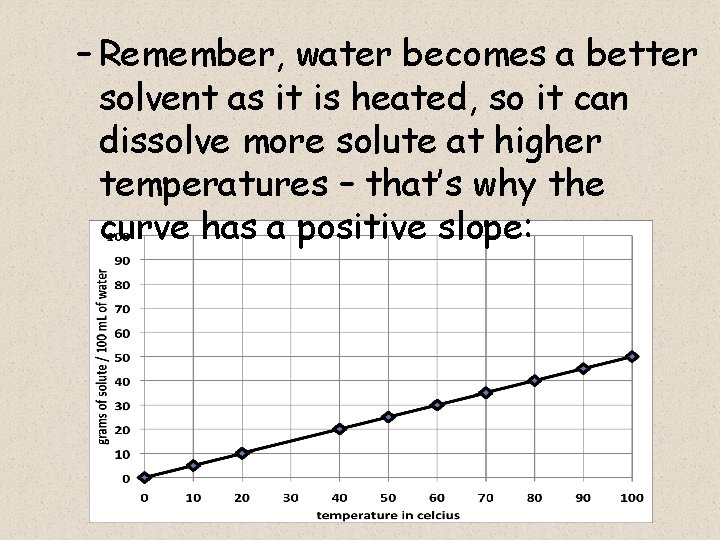
– Remember, water becomes a better solvent as it is heated, so it can dissolve more solute at higher temperatures – that’s why the curve has a positive slope:

• The LINE ITSELF also represents a “perfect” SOLUTION; that is, a solution with the EXACT amount of solute that will saturate 100 m. L of water:

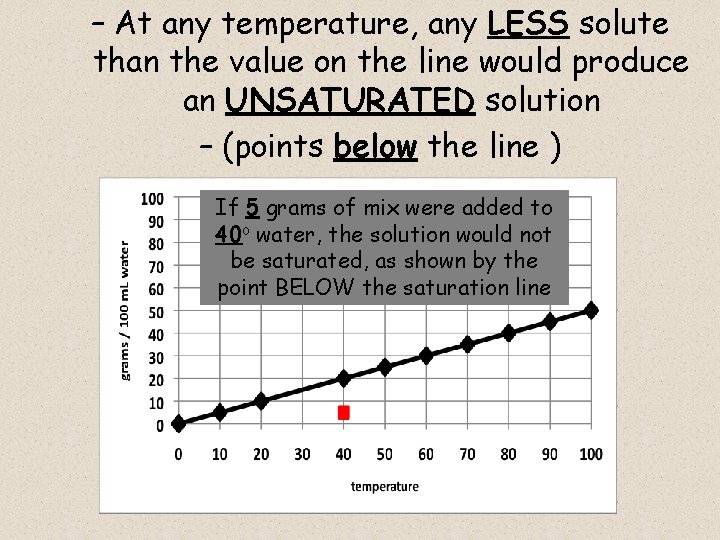
– At any temperature, any LESS solute than the value on the line would produce an UNSATURATED solution – (points below the line ) If 5 grams of mix were added to 40 o water, the solution would not be saturated, as shown by the point BELOW the saturation line

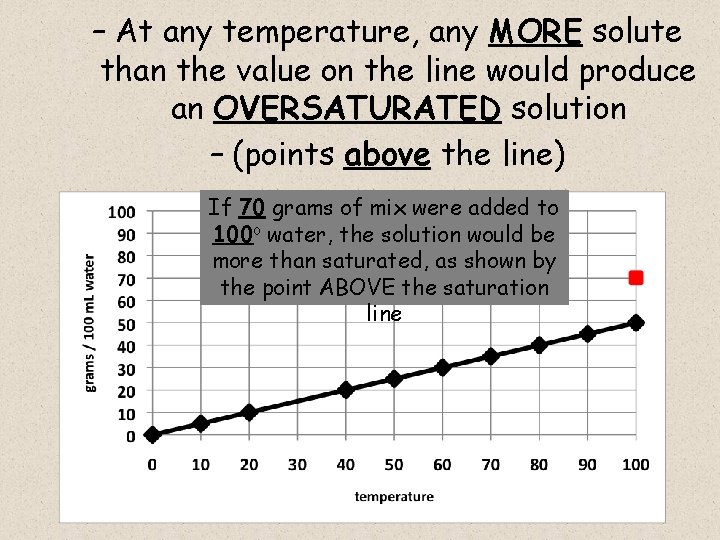
– At any temperature, any MORE solute than the value on the line would produce an OVERSATURATED solution – (points above the line) If 70 grams of mix were added to 100 o water, the solution would be more than saturated, as shown by the point ABOVE the saturation line

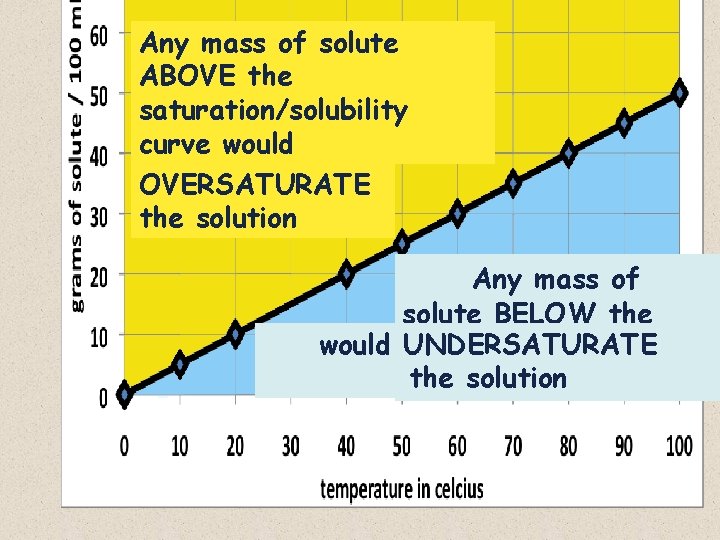
Any mass of solute ABOVE the saturation/solubility curve would OVERSATURATE the solution Any mass of solute BELOW the would curve UNDERSATURATE the solution

UNDISSOLVED SOLUTE AT BOTTOM, or PRECIPITATE forms as the solution cools More solute can be dissolved to make the solution saturated

Vocabulary • Dissociating = to break apart ions – Na. Cl + H 2 O = Na+ separates from the Cl- and each are surrounded by water molecules • Dissolving = to break apart molecules – Sugar + H 2 O = molecules of sugar separate in water • Ionizing = another term for Dissociation

• Non electrolytes do not conduct electricity when dissolved in water. • Polar covalent molecules such as methanol CH 3 OH don’t fall apart into ions when they dissolve. • Weak electrolytes don’t fall completely apart into ions. • Strong electrolytes do ionizes completely.

Solutions are composed of: • Solvent = does the dissolving; always in the greater concentration • Solute = is dissolved; always in lesser concentration solvent concentration > solute concentration Ex. 5 g of Na. Cl + 1 Liter of water: – Solvent = – Solute =

Types of Solutions: • Saturated Solution: undissolved solute is in equilibrium with the dissolved solute • Unsaturated Solution: contains less than the saturated amt. of solute for that temperature • Supersaturated Solution: contains more solute than a saturated solution can normally hold.

Who will dissolve me? • “Like dissolves like” = polar solvent will dissolve polar solute or nonpolar solvent will dissolve nonpolar solute. – Ex. Salt will dissolve in water but not acetone. – Ex. Oil will not mix with water • Miscibility - the ability of two liquids to be mixed. – Oil is immiscible in water – Vinegar is miscible in water

Rate of Solution Affected By: • Surface area of solid exposed to solvent. • Increase SA, increase solution rate • Stirring of solution • Stirring the solution will prevent buildup of saturated solution around solute • Kinetic Energy (motion) of solute and solvent • Increase T = increase KE = Increase solution rate

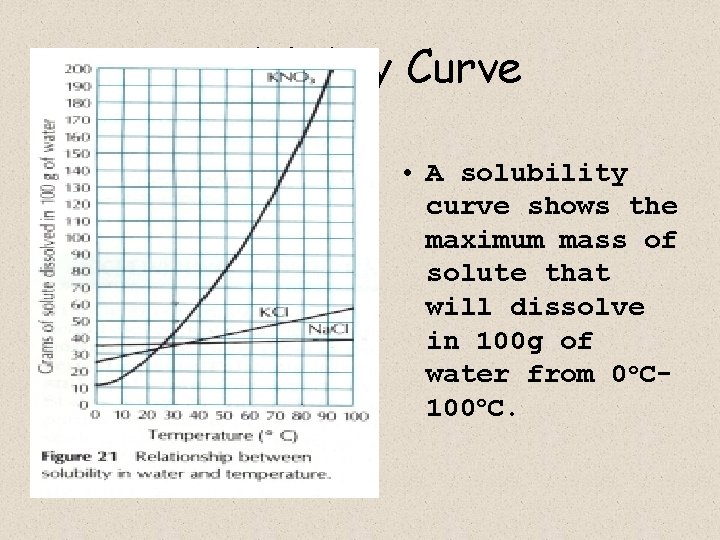
Solubility • The maximum mass of a substance that will dissolve in 100 g of water at a given temp. • Use solubility chart to determine. – EX: 40 g of Na. Cl dissolves in 100 g of water at 80 o. C.

Solubility Curve • A solubility curve shows the maximum mass of solute that will dissolve in 100 g of water from 0 o. C 100 o. C.

Types of Solutions • Saturated Solution: undissolved solute is in equilibrium with the dissolved solute (on the line) • Unsaturated Solution: contains less than the saturated amt. of solute for that temperature (below the line) • Supersaturated Solution: contains more solvent than a saturated solution can normally hold. (above the line)

. How many grams of potassium iodide can be dissolved in 100 g of water at 700 C? 2. 100 g of sodium nitrate in 100 g of water at 50 o. C is a(n) _______ solution. 3. 190 g of sodium nitrate in 100 g of water at 80 o. C is a(n) _______ solution. 4. How many grams of potassium nitrate make a saturated solution at 70 o. C? 5. Describe an unsaturated solution of potassium chlorate. 1