Grade 7 Science Unit 3 Mixtures Solutions Chapter




























- Slides: 37

Grade 7 Science Unit 3: Mixtures & Solutions Chapter 7 Matter can be classified as mixtures or pure substances

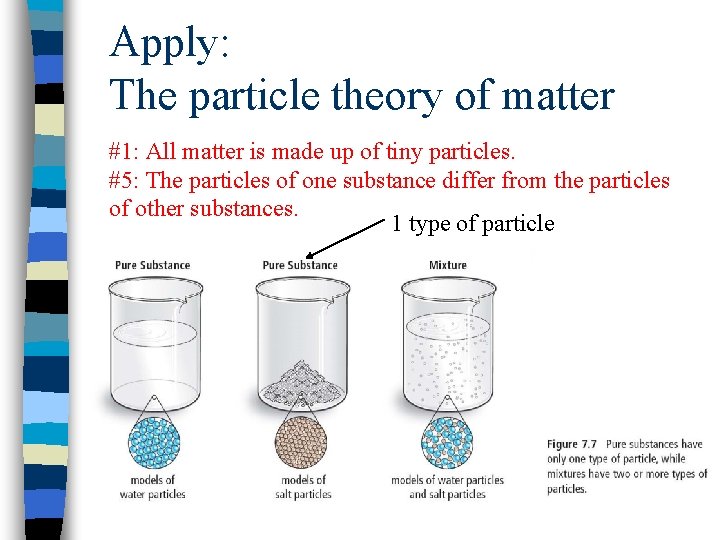
Particle Theory of Matter 1. 2. 3. 4. 5. All matter is made up of tiny particles. These particles are always moving… they have energy. There are spaces among particles. There attractive forces between the particles. The particles of one substance differ from the particles of other substances.

7. 1 Page 230 HOW MIXTURES ARE DIFFERENT FROM PURE SUBSTANCES

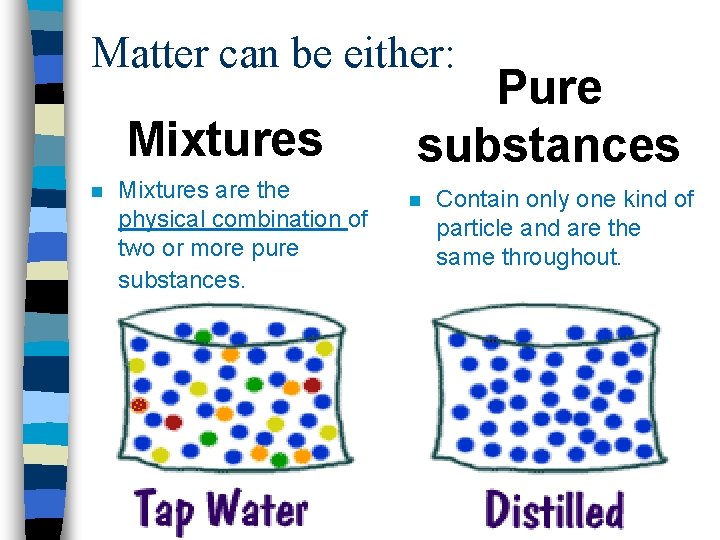
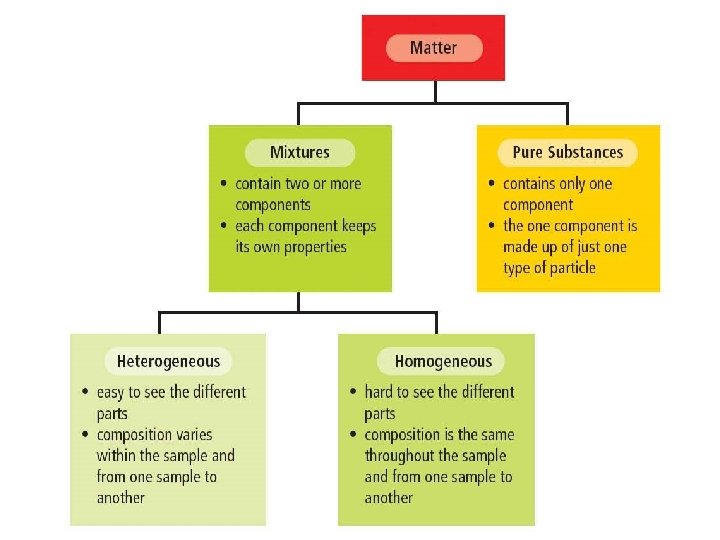
Matter can be either: Mixtures n Mixtures are the physical combination of two or more pure substances. Pure substances n Contain only one kind of particle and are the same throughout.

Pure Substances. . . p. 236 ALWAYS appear as uniform throughout n They contain either: n 1. Only one type of particle • Gold and Oxygen. 2. Two or more particles chemically combined to form a different substance. • Water is H²O which is 2 hydrogen's and 1 oxygen

Pure Substance n Substances don’t usually occur in their pure form in nature, so in order to obtain pure substances, people must refine raw materials. Bauxite makes Aluminum foil

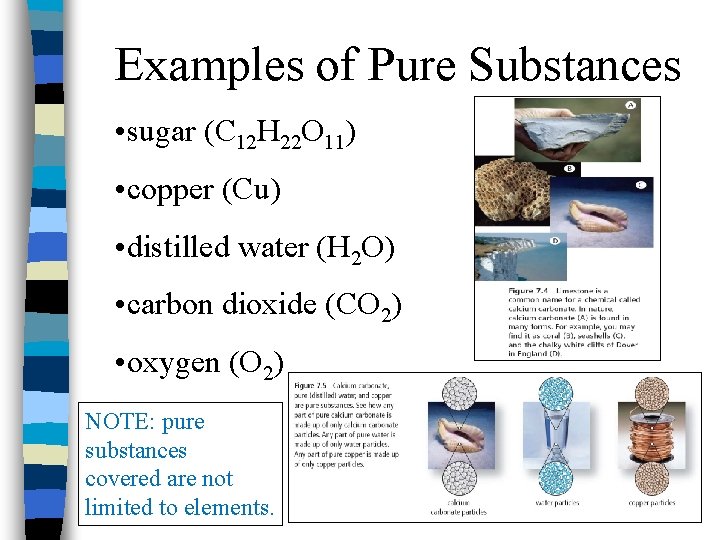
Examples of Pure Substances • sugar (C 12 H 22 O 11) • copper (Cu) • distilled water (H 2 O) • carbon dioxide (CO 2) • oxygen (O 2) NOTE: pure substances covered are not limited to elements.


Mixtures n They are the physical combination of two or more pure substances. + Sugar = Water ?

Mixtures p. 232 MAY have distinct visible components. n MAY appear uniform throughout. n

Six of the possible kinds of mixtures are: A. B. C. D. E. F. a mixture of gases a mixture of liquids a mixture of gases in a liquid a mixture of solids and gases

Soft drinks are mixtures made from: - liquid water - Solid sugar - Carbon dioxide gas

Commonly used mixtures n n Detergents Cleaners – Formed by adding water to the cleaner n Gasoline – oil mixtures – Lawn mower engines require specific amounts of oil to be mixed with gas

Examples of Mixtures… • kool-aid • chocolate chip cookie • concrete • salad dressing • Air • Bread

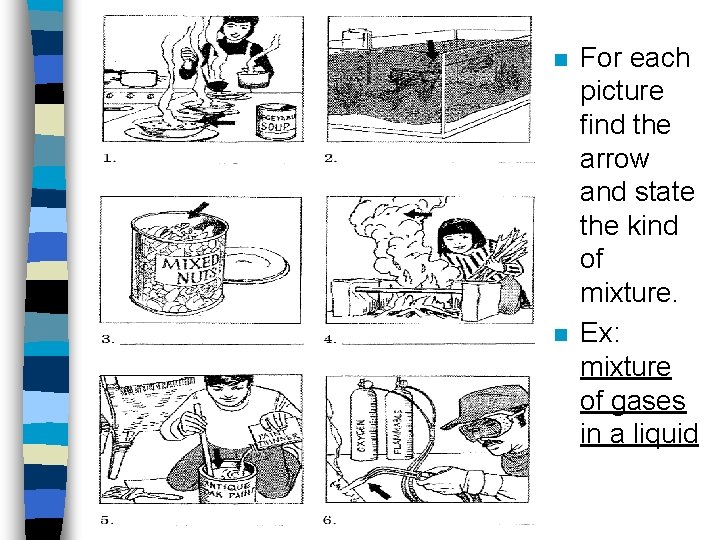
n n For each picture find the arrow and state the kind of mixture. Ex: mixture of gases in a liquid

Apply: The particle theory of matter #1: All matter is made up of tiny particles. #5: The particles of one substance differ from the particles of other substances. 1 type of particle

In class activity True. False n 1. Air is a mixture. _____ n 2. Gold is a pure substance. _____ n 3. Water from a lake is a pure substance. _____ n 4. A pure substance contains particles that are all alike. _____ n 5. Two different gases together make up a mixture. _____ n Work book: – Page 1 – 3

p. 235 Heterogeneous and homogeneous MIXTURES


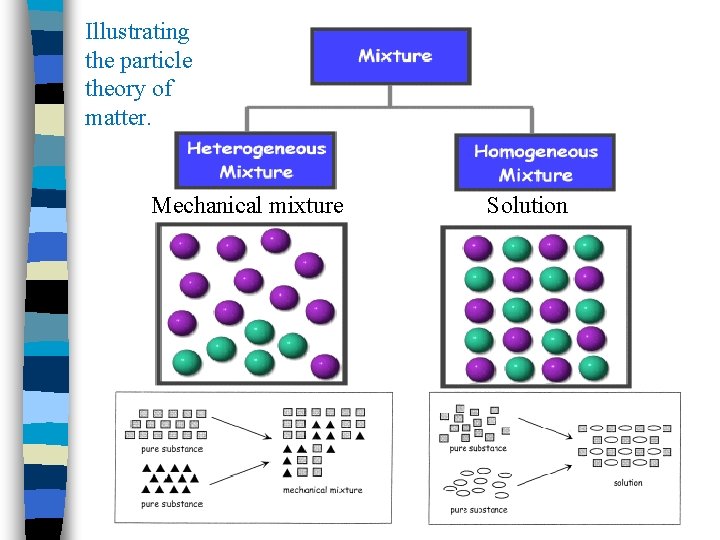
What is a mechanical Mixture? n Mixtures composed of two or more substances that remain visible even after they are mixed. n Sometimes need a microscope (see p. 245) n Can be solid, liquid or gas n The particles do not evenly mix

n Mechanical mixtures are also known as heterogeneous p. 244 mixtures (uneven mixtures). – A salad dressing made of oil and vinegar is considered a heterogeneous mixture because its components: • oil and vinegar — remain separate and distinct. – Think about a chocolate chip cookie. • Would it be considered a mechanical mixture? • Why or why not? ________________________________

Heterogeneous Mixtures n Example: – Smog-filled air • the amount of smog is not uniform inside the air. – Granola cereal or trail mix • the individual ingredients such as raisins, nuts, and dried fruit remain visible even after mixing. – Concrete • the components of the sand, lime, and water used to create it often remain visible.

Heterogeneous Mixtures Granola bar

What is a Solution? A type of mixture in which the pure substances within the solution mix together so well that they give the appearance of only one substance. n Solutions can be any combination of the three states of matter— solid, liquid, and gas. n Particles appear to be mixed evenly and to be the same throughout. n

p. 243 n Solutions are also called Homogeneous mixtures n Examples: – When sugar is mixed with water, the sugar seems to "disappear. " Yet, if you were to taste the mixture, you would know the sugar was still there. Also, if you were to place the solution in a shallow dish and allow the water to evaporate, the sugar would reappear. – Air - dissolve carbon dioxide, oxygen, and argon into nitrogen gas. – Brass- made up of copper and zinc.

Homogeneous Mixture n The particles are evenly mixed so that none of the original substances are visible. Kool-aid Stainless steel

Mixtures can be classified into 2 types: 1. 2. Heterogeneous – A non-uniform mixing • Particles create layers or parts • May also appear as one substance • Light will scatter as it passes through • May or may not need a microscope to see parts Homogeneous – A uniform mixing • Appear as one substance • Particles are evenly spread out • Light will pass through unaffected • Cannot see parts with a microscope p. 234

Illustrating the particle theory of matter. Mechanical mixture Solution

p. 235 Questions 1 – 3 p. 237 Questions 1 – 3 p. 241 Questions 1 - 6 Workbook IN CLASS WORK

Combination of mixtures n p. 244 Sometimes you cannot tell whether something is homogeneous or heterogeneous just by looking at it. – The mixtures are hard to classify: • Homogeneous • Heterogeneous However, we can use light to determine… n

Using light: homogeneous and heterogeneous http: //www. youtube. com/watch? v=Na. URE 8 g. TXqk

Homogeneous Mixture • • They appear to be ONE substance light passes through unaffected

Heterogeneous Mixtures n Light will scatter because it reflects off the particles

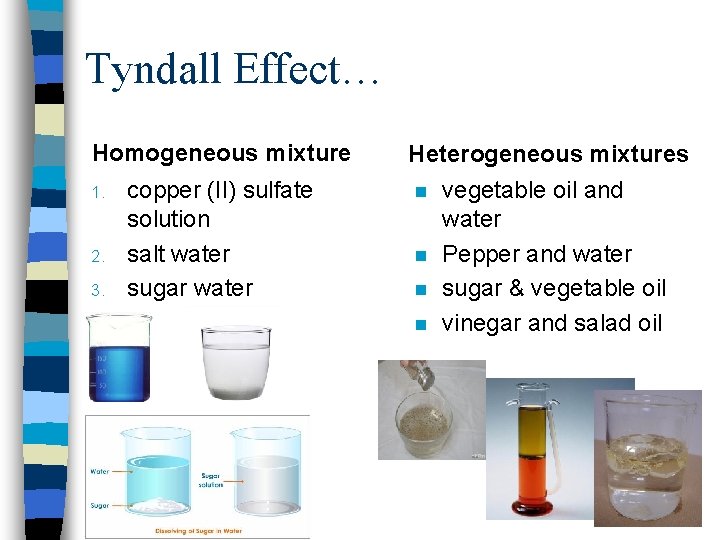
Tyndall Effect… Homogeneous mixture 1. 2. 3. copper (II) sulfate solution salt water sugar water Heterogeneous mixtures n n vegetable oil and water Pepper and water sugar & vegetable oil vinegar and salad oil

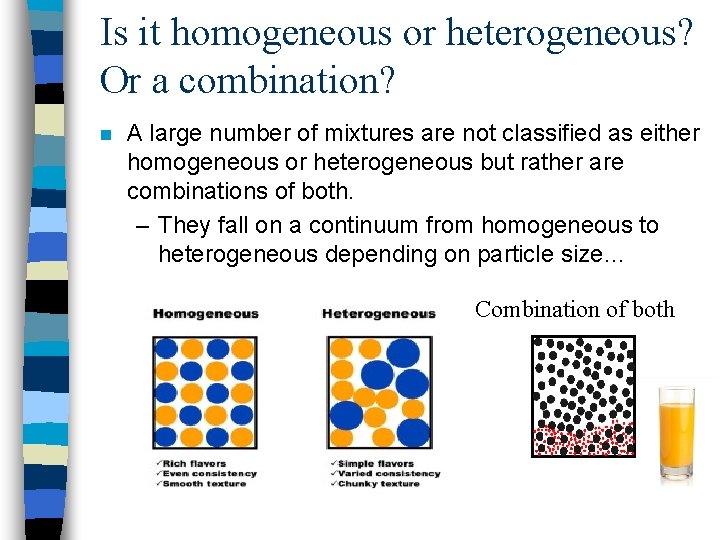
Is it homogeneous or heterogeneous? Or a combination? n A large number of mixtures are not classified as either homogeneous or heterogeneous but rather are combinations of both. – They fall on a continuum from homogeneous to heterogeneous depending on particle size… Combination of both

Some mixtures are combinations of heterogeneous and homogeneous mixtures n Milk Orange juice Soft drink n Ex: Orange juice: n n – Homogeneous: sugars with water – Heterogeneous: is a combination of solid orange pulp and water.

In class activity n Name one example of a heterogeneous mixture and one example of a homogeneous mixture. – Explain how you are able to tell the two types of mixtures apart. n n Chapter review p. 250 Questions 1 -10 p. 245 Questions 1 -4 p. 249 Questions 1 -6 Workbook