Water Potential Plants water potential Plants can use




![Hints & reminders 1. Remember water always moves from [hi] to [lo]. 2. Water Hints & reminders 1. Remember water always moves from [hi] to [lo]. 2. Water](https://slidetodoc.com/presentation_image_h2/445b87dddf32337ed151a5ab3e1964ee/image-12.jpg)
- Slides: 12

Water Potential

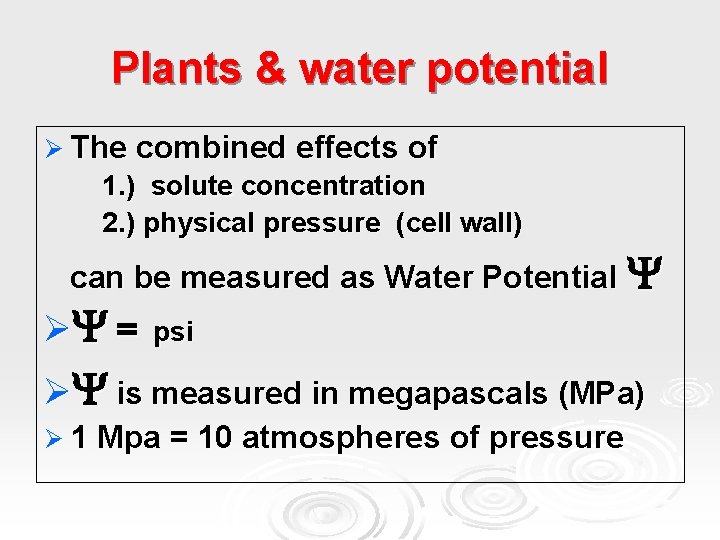
Plants & water potential Plants can use the potential energy in water to perform work. Ø Tomato plant regains turgor pressure – cell pushes against wall due to uptake of water Ø

Plants & water potential Ø The combined effects of 1. ) solute concentration 2. ) physical pressure (cell wall) can be measured as Water Potential Ø = psi Ø is measured in megapascals (MPa) Ø 1 Mpa = 10 atmospheres of pressure

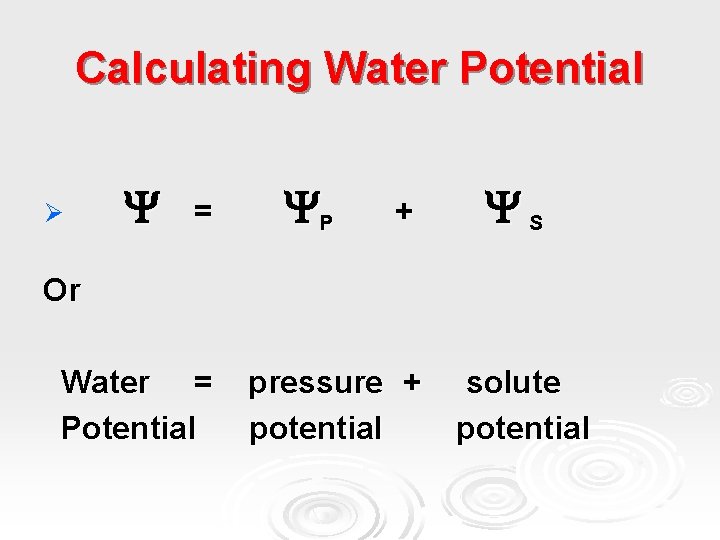
Calculating Water Potential Ø = P + S Or Water = Potential pressure + potential solute potential

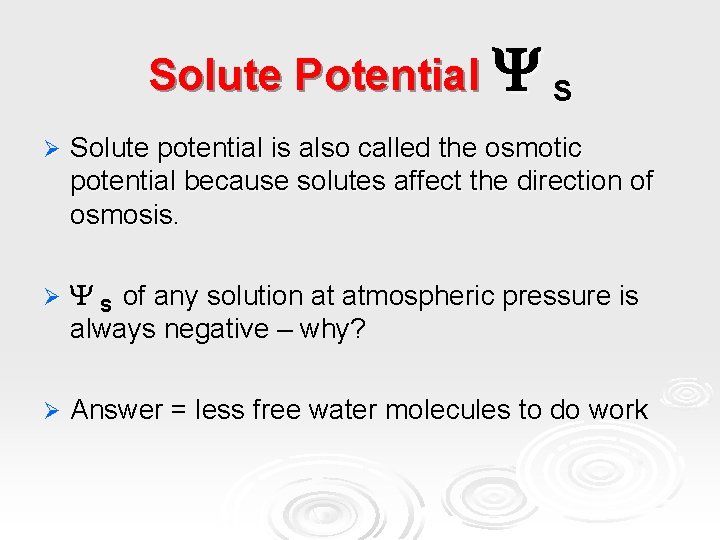
Solute Potential S Ø Solute potential is also called the osmotic potential because solutes affect the direction of osmosis. Ø S of any solution at atmospheric pressure is always negative – why? Ø Answer = less free water molecules to do work

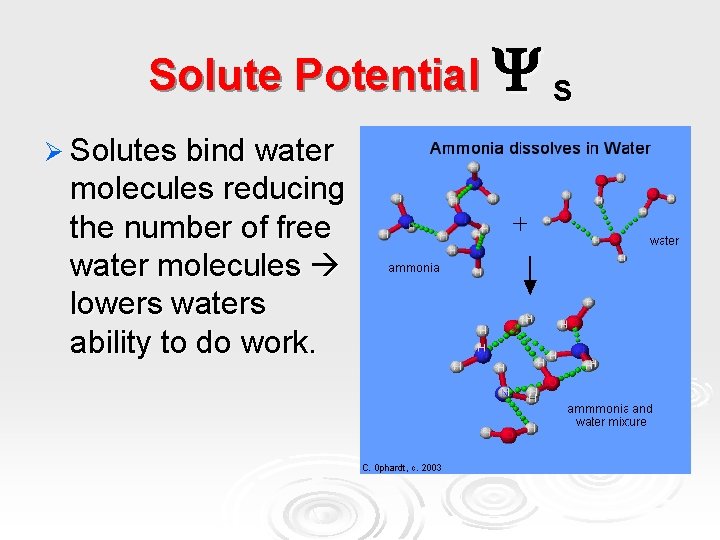
Solute Potential S Ø Solutes bind water molecules reducing the number of free water molecules lowers waters ability to do work.

Pressure Potential P Ø P is the physical pressure on a solution. Ø P can be negative transpiration in the xylem tissue of a plant (water tension) Ø P can be positive water in living plant cells is under positive pressure (turgid)

Standard for measuring Ø Pure water is the standard. Ø Pure water in an open container has a water potential of zero at one atmosphere of pressure.

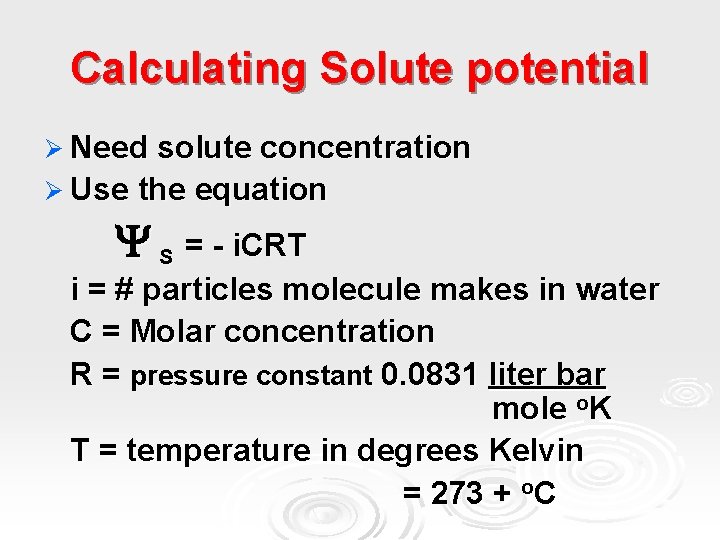
Calculating Solute potential Ø Need solute concentration Ø Use the equation S = - i. CRT i = # particles molecule makes in water C = Molar concentration R = pressure constant 0. 0831 liter bar mole o. K T = temperature in degrees Kelvin = 273 + o. C

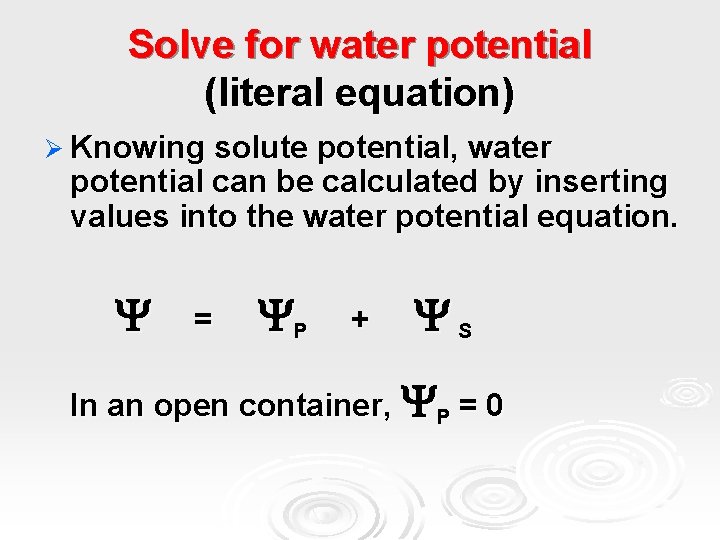
Solve for water potential (literal equation) Ø Knowing solute potential, water potential can be calculated by inserting values into the water potential equation. = P + S In an open container, P = 0

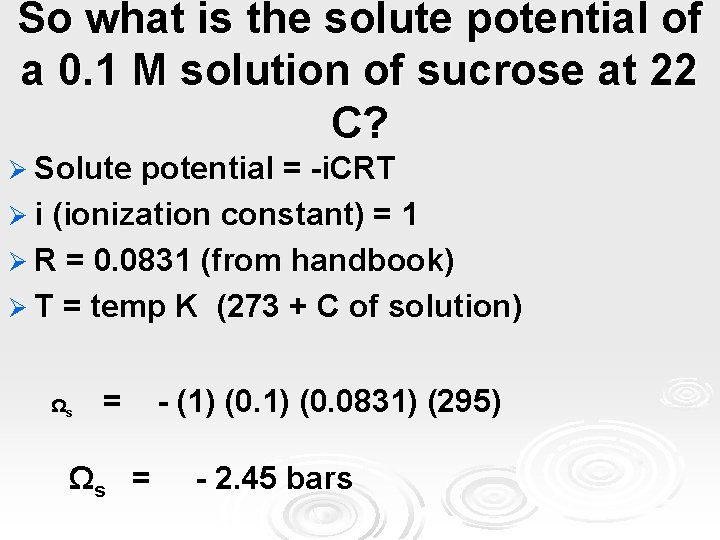
So what is the solute potential of a 0. 1 M solution of sucrose at 22 C? Ø Solute potential = -i. CRT Ø i (ionization constant) = 1 Ø R = 0. 0831 (from handbook) Ø T = temp K Ωs = (273 + C of solution) - (1) (0. 0831) (295) - 2. 45 bars
![Hints reminders 1 Remember water always moves from hi to lo 2 Water Hints & reminders 1. Remember water always moves from [hi] to [lo]. 2. Water](https://slidetodoc.com/presentation_image_h2/445b87dddf32337ed151a5ab3e1964ee/image-12.jpg)
Hints & reminders 1. Remember water always moves from [hi] to [lo]. 2. Water moves from hypo hypertonic. 3. [Solute] is related to osmotic pressure. Pressure is related to pressure potential. 4. Pressure raises water potential. 5. When working problems, use zero for pressure potential in animal cells & open beakers. 6. 1 bar of pressure = 1 atmosphere