Unit 14 Part 1 Solutions Part 2 Acids


































![Definition: Hydronium Ion In aqueous solution, H+ does NOT exist! Note: In problems, [H+] Definition: Hydronium Ion In aqueous solution, H+ does NOT exist! Note: In problems, [H+]](https://slidetodoc.com/presentation_image_h/2babefde0c3b6d173d160836c26f1e92/image-51.jpg)
![p. H n p. H = - log [H 3 O+] p. OH = p. H n p. H = - log [H 3 O+] p. OH =](https://slidetodoc.com/presentation_image_h/2babefde0c3b6d173d160836c26f1e92/image-52.jpg)




- Slides: 60

Unit 14 Part 1: Solutions Part 2: Acids and Bases

Solutions All solutions are composed of two parts: The solute and the solvent. The substance that gets dissolved is the solute The substance that does the dissolving is the solvent (Usually present in the larger amount) ***A solution may exist as a solid, liquid or gas depending on the state of the solvent.

Types of Solutions n Gas in liquid Example – soda water n Solute: carbon dioxide (gas) Solvent: water (liquid) n Solid in solid Example – Steel n Solute: carbon Solvent: iron

Types of Solutions n Gas in gas Example - Air n Solute: Oxygen Solvent: Nitrogen

Types of Solutions n Liquid in liquid Example – Vinegar n Solute: Acetic acid Solvent: Water n Solid in liquid Example – Ocean Water n Solute: Sodium Chloride (solid) Solvent: Water (liquid)

Aqueous Solution n Any mixture where water is the solvent. n Something is dissolved in water

Solubility Soluble - a substance that dissolves in another substance. Insoluble - a substance that does not dissolve in another substance.

Solubility Immiscible - two liquids that are insoluble in each other. Miscible - two liquids that are soluble in each other.

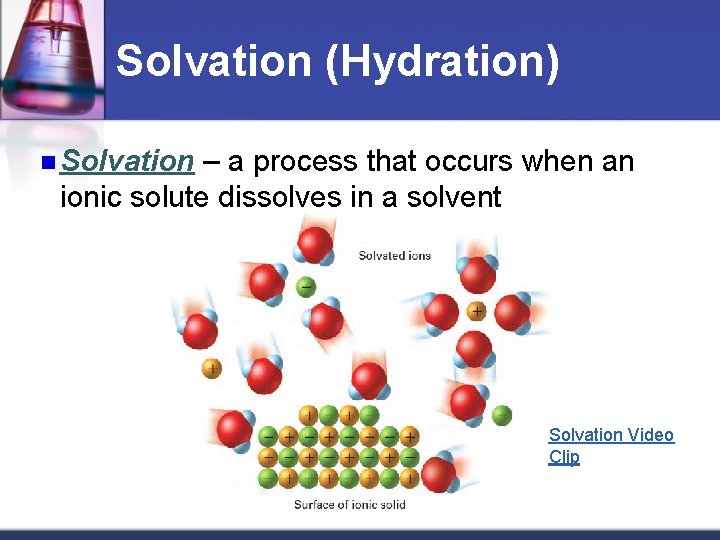
Solvation (Hydration) n Solvation – a process that occurs when an ionic solute dissolves in a solvent Solvation Video Clip

“Like Dissolves Like” Solvents of a specific polarity or type will dissolve solute of similar polarities or types! (Polar things dissolve in other polar things) Examples:

Solubility n. Solubility – the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature and pressure to produce a saturated solution • Units for solubility: grams of solute per 100 g solvent Example: At 20˚C, Na. NO 3 has a solubility of 74 g/100 g H 2 O

Solubility n Saturated Solution - contains the maximum amount of dissolved solute n Unsaturated Solution - contains less than the maximum amount of dissolved solute n Supersaturated Solution – contains more solute than can theoretically be dissolved at a given temperature

Supersaturated Solutions n. How can you dissolve more solute than possible? ?

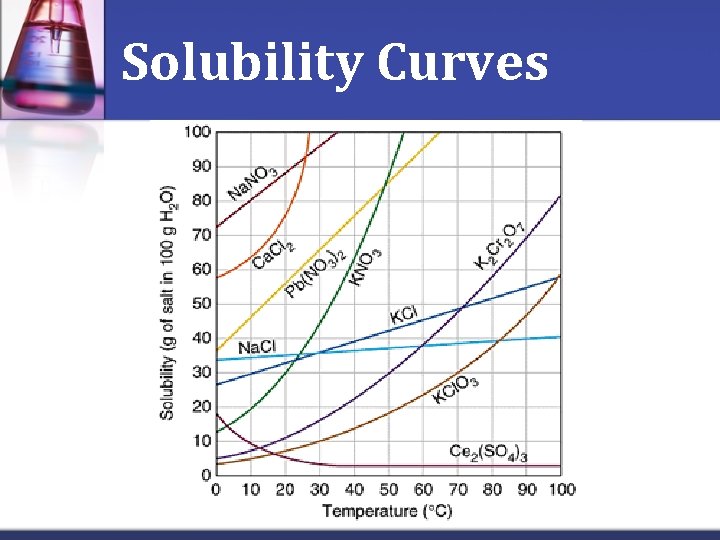
Solubility Curves n. Solubility of a solid generally increases with increasing temperature n. The higher the temperature, the greater amount of solute that will be dissolved in the solvent n. Solubility can be represented in a chart called a solubility curve

Solubility Curves

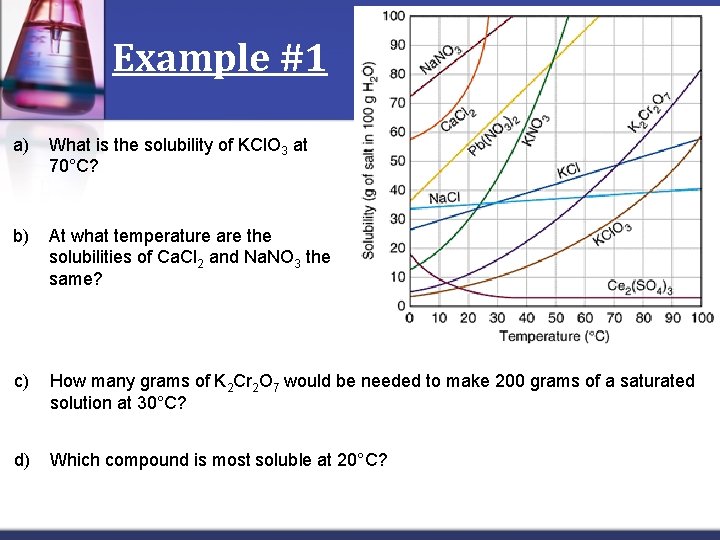
Example #1 a) What is the solubility of KCl. O 3 at 70°C? b) At what temperature are the solubilities of Ca. Cl 2 and Na. NO 3 the same? c) How many grams of K 2 Cr 2 O 7 would be needed to make 200 grams of a saturated solution at 30°C? d) Which compound is most soluble at 20°C?

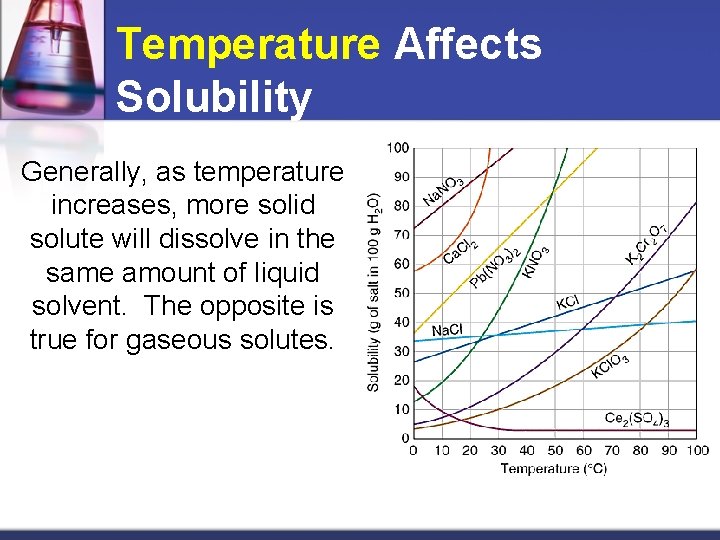
Temperature Affects Solubility Generally, as temperature increases, more solid solute will dissolve in the same amount of liquid solvent. The opposite is true for gaseous solutes.

CONCENTRATION n Concentration – the measure of the amount of solute dissolved in a given quantity of solvent

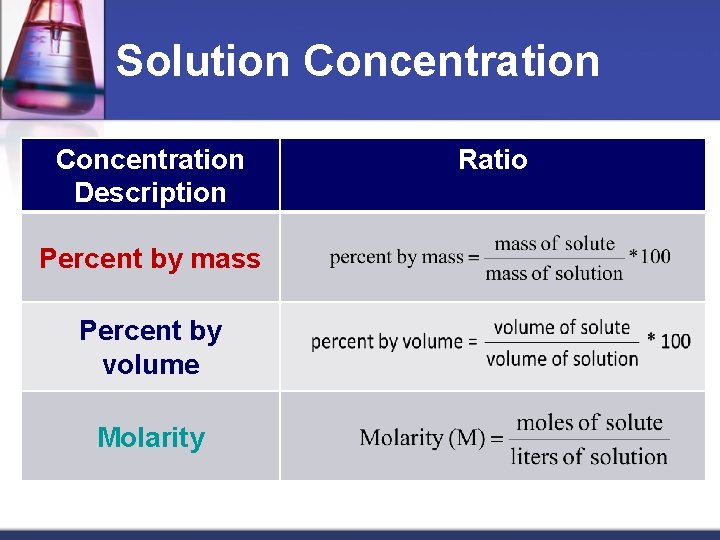
Solution Concentration Description Percent by mass Percent by volume Molarity Ratio

Example #2 An aquarium contains 3. 6 g Na. Cl per 100. 0 g of water. What is the percent by mass of Na. Cl in the solution?

Example #3 What is the percent by volume of ethanol in a solution that contains 35 m. L of ethanol dissolved in 115 m. L of water?

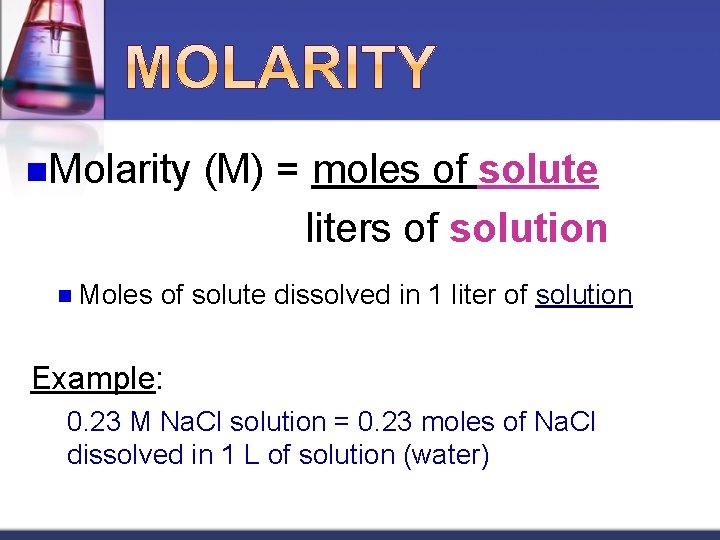
n. Molarity n Moles (M) = moles of solute liters of solution of solute dissolved in 1 liter of solution Example: 0. 23 M Na. Cl solution = 0. 23 moles of Na. Cl dissolved in 1 L of solution (water)

n. M is read as “molar” when next to a number n 4 M HCl = 4 molar hydrochloric acid n Keep in mind that the liters of solution takes into account the volume of the solute AND the volume of the solvent

Example #4 What is the molarity of a solution that contains 0. 65 mol of Cu. Cl 2 in 500 m. L of water?

Example #5 What is the molarity of a solution that contains 5. 10 g of glucose (C 6 H 12 O 6) in 100. 5 m. L of solution?

Preparing Molar Solutions How do you make a solution with a specific molarity? 1. Convert moles of solute to grams and measure the amount out. 2. Add solvent so that the total volume of the solution is 1 L. 3. For any volume other than 1 L we must adjust the amount of solute needed by multiplying it by the fraction of a liter of solution we need.

Example #6 How many grams of Ca. Cl 2 would need to be dissolved in 1. 0 L of water to make a 0. 10 M solution of Ca. Cl 2?

Example #7 How many grams of Na. OH are in 250 m. L of a 3. 0 M Na. OH solution?

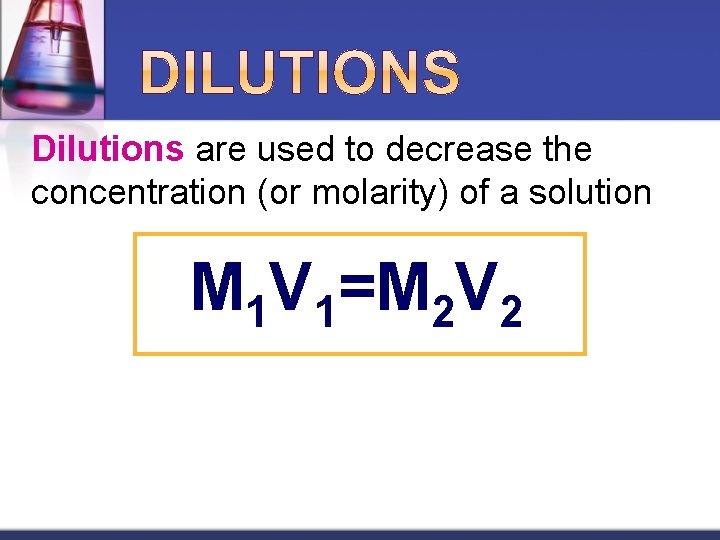
Dilutions are used to decrease the concentration (or molarity) of a solution M 1 V 1=M 2 V 2

Steps to Performing a Dilution 1. Calculate how many m. L of the original (stock) solution to start with 2. Measure out the volume of stock solution (using a graduated cylinder or a pipet) and place in appropriately sized volumetric flask 3. Add water to the mark on flask


Example #8 What volume (in m. L) of 2. 00 M Ca. Cl 2 is needed to make 0. 50 L of 0. 300 M Ca. Cl 2 solution?

Example #9 What volume of water would you add to 15. 00 m. L of a 6. 77 M solution of nitric acid (HNO 3) in order to get a 1. 50 M solution?

Colligative Properties n n Colligative means “depending on the collection. ” n Depends only on the number of dissolved particles, not on the identity of dissolved particles. Examples of colligative properties: n boiling point elevation and freezing point depression

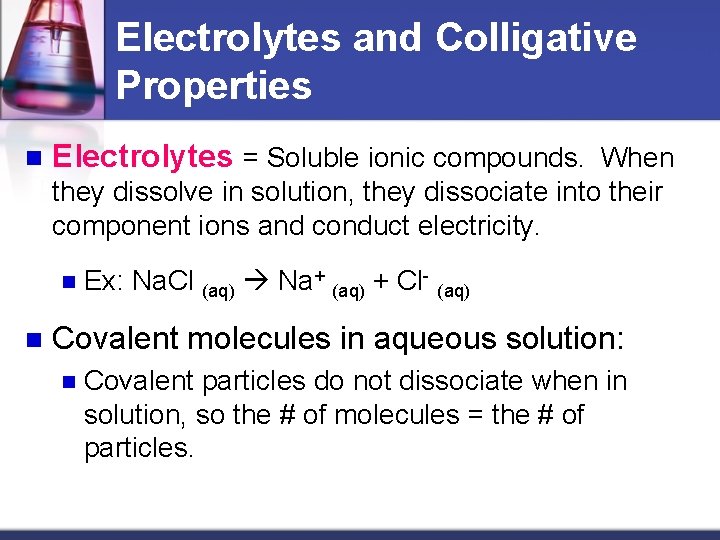
Electrolytes and Colligative Properties n Electrolytes = Soluble ionic compounds. When they dissolve in solution, they dissociate into their component ions and conduct electricity. n n Ex: Na. Cl (aq) Na+ (aq) + Cl- (aq) Covalent molecules in aqueous solution: n Covalent particles do not dissociate when in solution, so the # of molecules = the # of particles.

Examples of Colligative Properties Boiling Point Elevation n Boiling occurs when vapor pressure equals atmospheric pressure. n Boiling point of a solution is higher than the boiling point of the pure solvent. n Dissolving substances increases the boiling point of a solvent.

Freezing Point Depression n Freezing point of a solution is lower than the freezing point of the pure solvent. n n Dissolving substances lowers the freezing point of a solvent. Ex: Icy pavement - throw down Ca. Cl 2 or Na. Cl, and the water will then freeze at a lower temperature

Ex: Antifreeze: a solution of ethylene glycol in water Prevents car’s radiator from freezing in the winter. 2. Prevents car’s radiator from boiling over in the summer. The more ethylene glycol in the water, the lower the freezing point, and the higher the boiling point. 1.

Part 2 Acids & Bases

Properties of Acids Physical Properties: n Taste sour Chemical Properties: n n React with metals to produce H 2 gas Neutralized when reacted with a base Litmus Indicator: n Turns blue litmus paper red Ions in Solution: n H+, H 3 O+ (hydronium ion)

Properties of Bases Physical Properties: n n Taste Bitter Slippery Chemical Properties: n n n Neutralized when reacted with an acid Do not react with metals Why are bases used as drain cleaners? Litmus Indicator: n Turns red litmus paper blue Ions in Solution: n OH-1

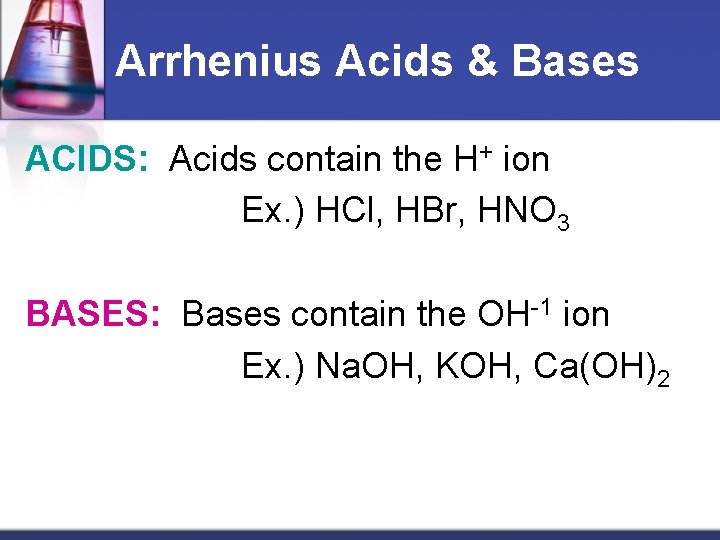
Arrhenius Acids & Bases ACIDS: Acids contain the H+ ion Ex. ) HCl, HBr, HNO 3 BASES: Bases contain the OH-1 ion Ex. ) Na. OH, KOH, Ca(OH)2

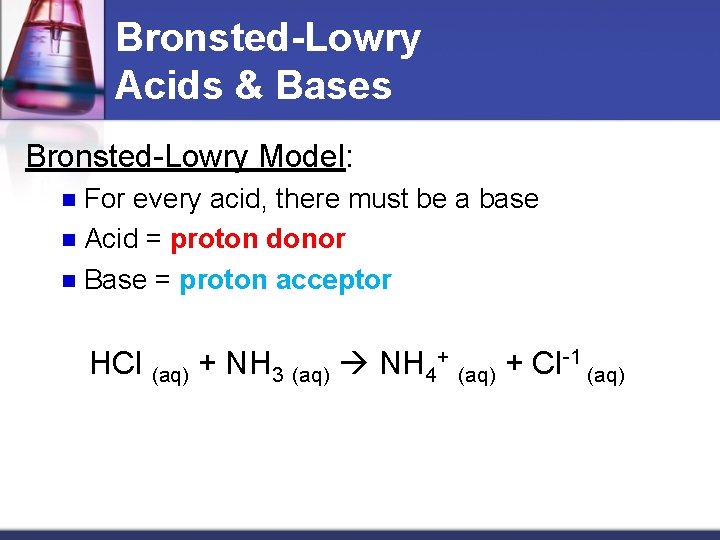
Bronsted-Lowry Acids & Bases Bronsted-Lowry Model: For every acid, there must be a base n Acid = proton donor n Base = proton acceptor n HCl (aq) + NH 3 (aq) NH 4+ (aq) + Cl-1 (aq)

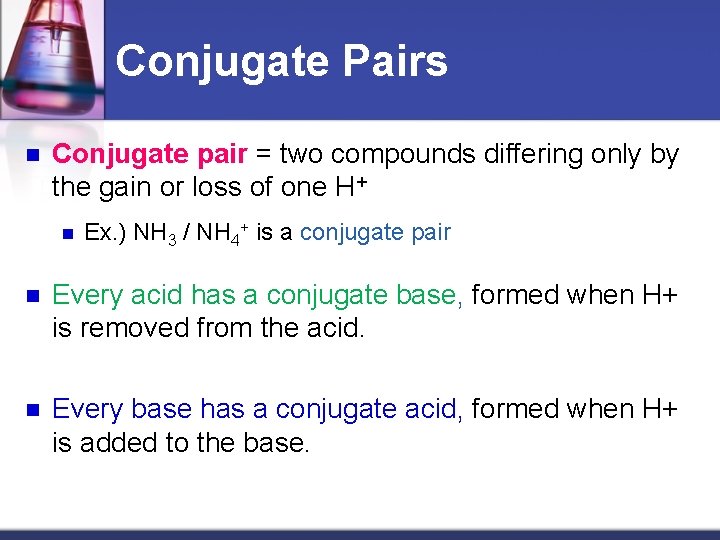
Conjugate Pairs n Conjugate pair = two compounds differing only by the gain or loss of one H+ n Ex. ) NH 3 / NH 4+ is a conjugate pair n Every acid has a conjugate base, formed when H+ is removed from the acid. n Every base has a conjugate acid, formed when H+ is added to the base.

Example #10 Write the reaction & identify the conjugate pair of each compound: a) HCl in water b) NH 3 in water c) HSO 4 - and NH 3

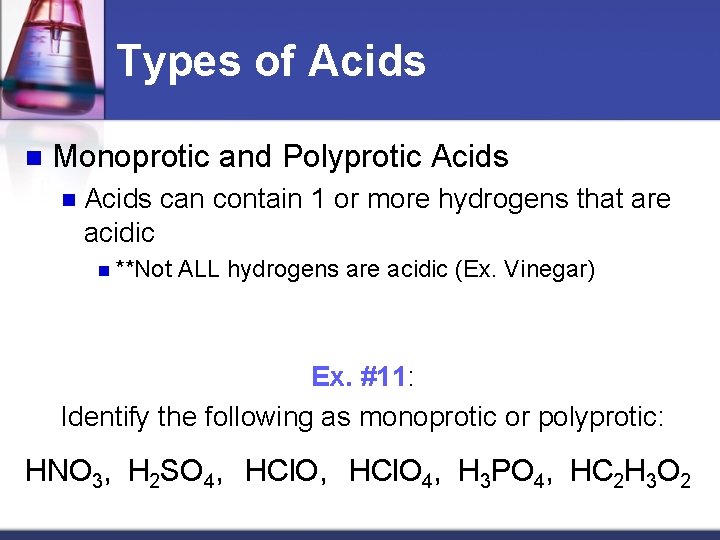
Types of Acids n Monoprotic and Polyprotic Acids n Acids can contain 1 or more hydrogens that are acidic n **Not ALL hydrogens are acidic (Ex. Vinegar) Ex. #11: Identify the following as monoprotic or polyprotic: HNO 3, H 2 SO 4, HCl. O 4, H 3 PO 4, HC 2 H 3 O 2

Strength of Acids/Bases Strengths of Acids n Strong Acid Give off LOTS of H+ n 100% Dissociation n Strong n That’s n Acids: HCl, HI, HBr, HNO 3, H 2 SO 4, HCl. O 4 it! Everything else is “weak” Weak Acid Give off smaller amounts of H+ n Equilibrium occurs (molecules break apart and recombine) n Not all H+ ions separate (not 100% dissociation)

Strength of Acids/Bases Strengths of Bases n Strong Base Give off LOTS of OH-1 n 100% Dissociation n Generally, Group I, II Hydroxides (except H, Be, Mg) n Ex. ) Ca(OH)2, Na. OH n Everything n else is “weak” Weak Base Give off smaller amounts of OH-1 n Equilibrium occurs (breaks apart and then recombines) n Not 100% dissociation

Strong or weak vs. concentrated and dilute n Strong/weak tells you how much the acid or base dissociates (breaks up) n Concentrated/dilute indicates the concentration (amount of solute in the solvent)

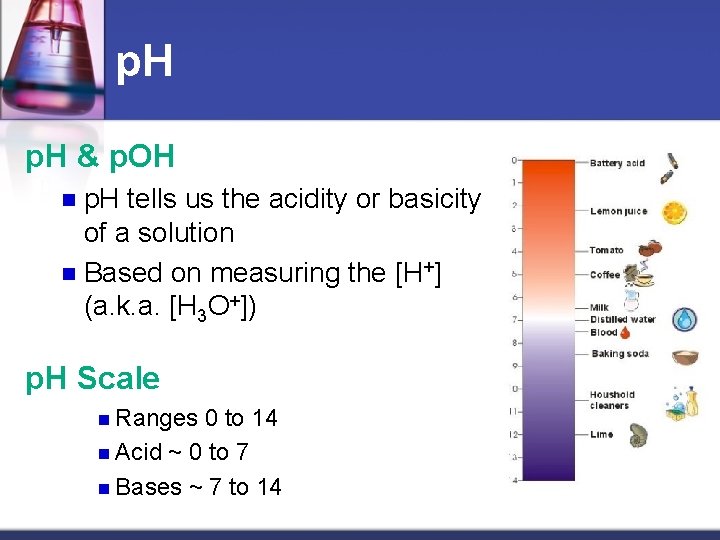
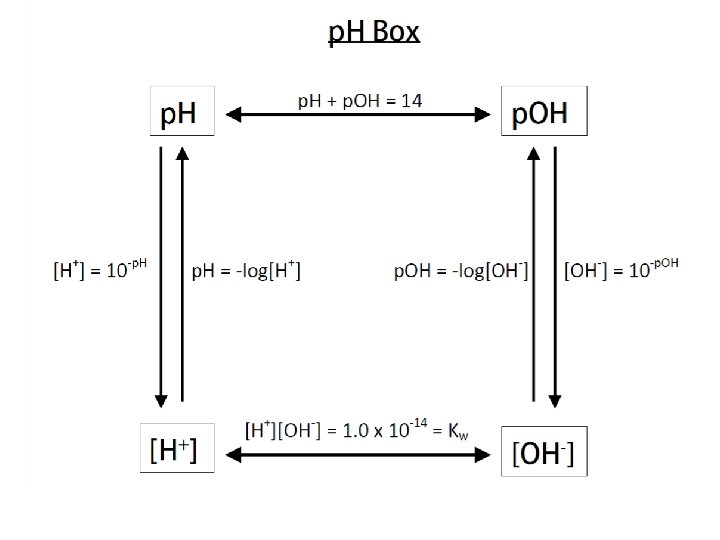
p. H & p. OH p. H tells us the acidity or basicity of a solution n Based on measuring the [H+] (a. k. a. [H 3 O+]) n p. H Scale n Ranges 0 to 14 n Acid ~ 0 to 7 n Bases ~ 7 to 14
![Definition Hydronium Ion In aqueous solution H does NOT exist Note In problems H Definition: Hydronium Ion In aqueous solution, H+ does NOT exist! Note: In problems, [H+]](https://slidetodoc.com/presentation_image_h/2babefde0c3b6d173d160836c26f1e92/image-51.jpg)
Definition: Hydronium Ion In aqueous solution, H+ does NOT exist! Note: In problems, [H+] = [H 3 O+] H + + H 2 O H 3 O + (hydronium ion)
![p H n p H log H 3 O p OH p. H n p. H = - log [H 3 O+] p. OH =](https://slidetodoc.com/presentation_image_h/2babefde0c3b6d173d160836c26f1e92/image-52.jpg)
p. H n p. H = - log [H 3 O+] p. OH = - log [OH-] n Make sure you have the negative sign! n Find the “log” function on your calculator! p. H + p. OH = 14 n [H+][OH-] = 1. 0 x 10 -14 n


Example #12 What is the p. H and p. OH for a solution with an H+ concentration, [H+], of 3. 0 x 10 -6 M H+?

Example #13 What is the H+ and OH- concentration of blood with a p. H of 7. 40?

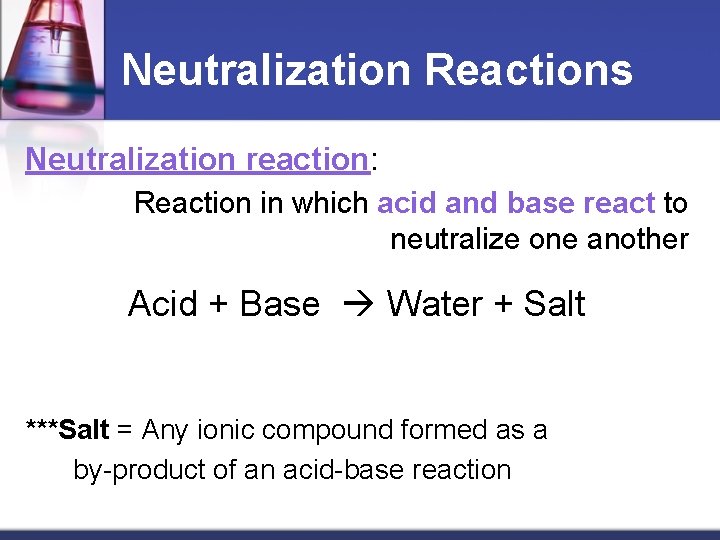
Neutralization Reactions Neutralization reaction: Reaction in which acid and base react to neutralize one another Acid + Base Water + Salt ***Salt = Any ionic compound formed as a by-product of an acid-base reaction

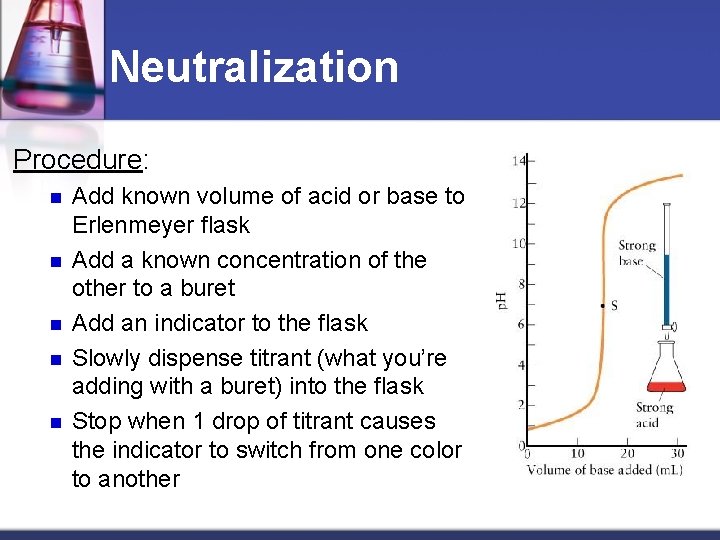
Neutralization Acid-base Titration: n Definition: Lab technique which allows you to get moles of acid and base EXACTLY equal to another n Complete neutralization n Allows you to calculate the concentration of an unknown acid or base n

Neutralization Procedure: n n n Add known volume of acid or base to Erlenmeyer flask Add a known concentration of the other to a buret Add an indicator to the flask Slowly dispense titrant (what you’re adding with a buret) into the flask Stop when 1 drop of titrant causes the indicator to switch from one color to another

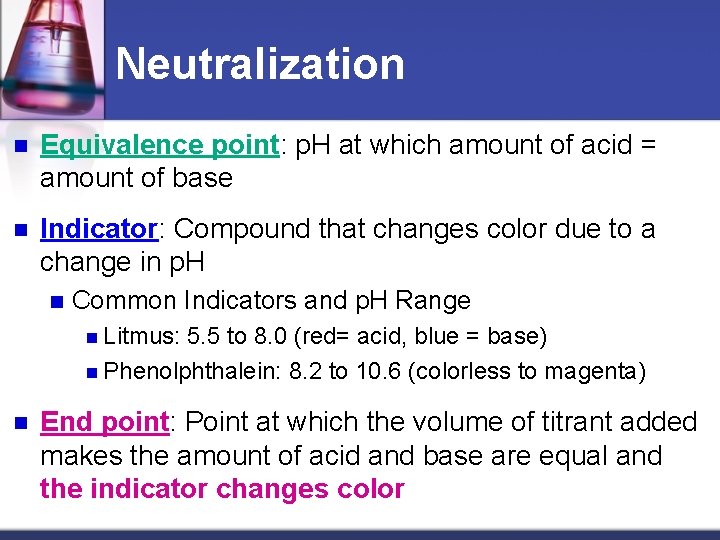
Definitions The titrant is the substance of known concentration used to determine the unknown concentration of the other substance. An indicator- substance that changes color at a certain p. H—is added to tell us when the neutralization is complete. Example: Phenolphthalein undergoes a color change between p. H 8 and 10 clear in acid Light pink in neutral Dark pink in base

Neutralization n Equivalence point: p. H at which amount of acid = amount of base n Indicator: Compound that changes color due to a change in p. H n Common Indicators and p. H Range n Litmus: 5. 5 to 8. 0 (red= acid, blue = base) n Phenolphthalein: 8. 2 to 10. 6 (colorless to magenta) n End point: Point at which the volume of titrant added makes the amount of acid and base are equal and the indicator changes color