AQUEOUS SOLUTIONS A solution is a homogeneous mixture





























- Slides: 63

AQUEOUS SOLUTIONS A solution is a homogeneous mixture of a solute dissolved in a solvent. The solvent is generally in excess. Example The solution Na. Cl(aq) is sodium chloride Na. Cl(s) dissolved in water H 2 O(l) The solute is Na. Cl(s) and the solvent is H 2 O(l)


Types of Solutions • Are solutions made from only one solvent and one solute? – By definition, there can only be one solvent – However, many solutes can be dissolved in a solvent to create a solution • Air is an example of a solution with one “solvent” (nitrogen) and many “solutes” (oxygen, helium, argon, carbon dioxide, etc. )

saturated sugar solution • Imagine there is a saturated sugar solution. (Saturated means that the maximum amount is dissolved in the solution, under normal conditions. ) There are undissolved sugar crystals at the bottom of the solution. This can be shown by the equation, • The equation describes that sugar crystals (sugar(s)) will dissolve in water (H 2 O) and produce sugar molecules in solution (sugar(aq)).

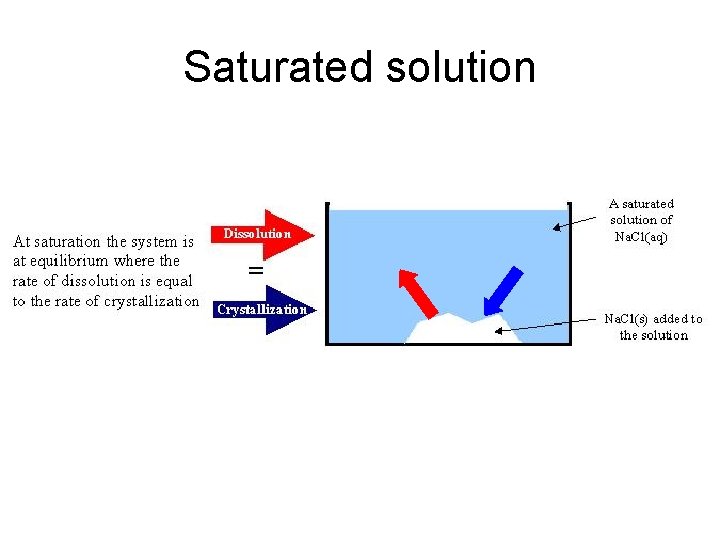
saturated sugar solution • Since the amount of sugar at the bottom does not change once equilibrium is attained, it would seem that the process stops. In other words, it seems that sugar does not go into solution or come out of solution anymore. • However, this is not true. The amounts of undissolved sugar crystals and sugar in solution do not change because the rate at which sugar molecules go into solution is the same rate as sugar molecules coming out of solution (forming crystals).

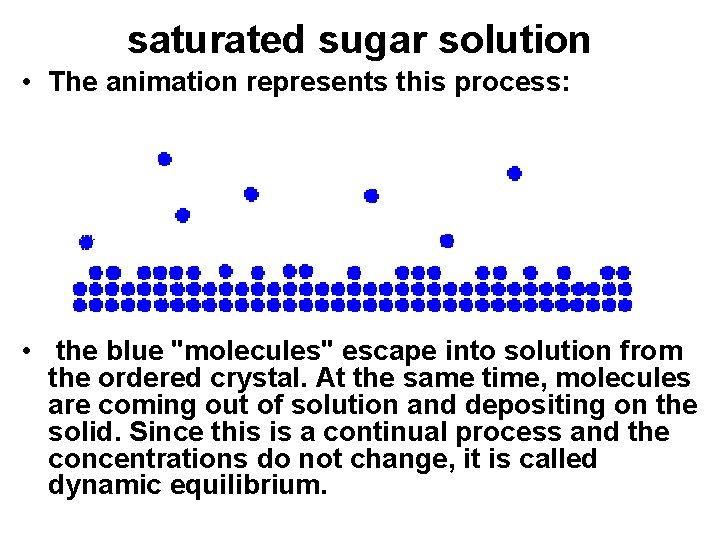
saturated sugar solution • The animation represents this process: • the blue "molecules" escape into solution from the ordered crystal. At the same time, molecules are coming out of solution and depositing on the solid. Since this is a continual process and the concentrations do not change, it is called dynamic equilibrium.

Saturated solution

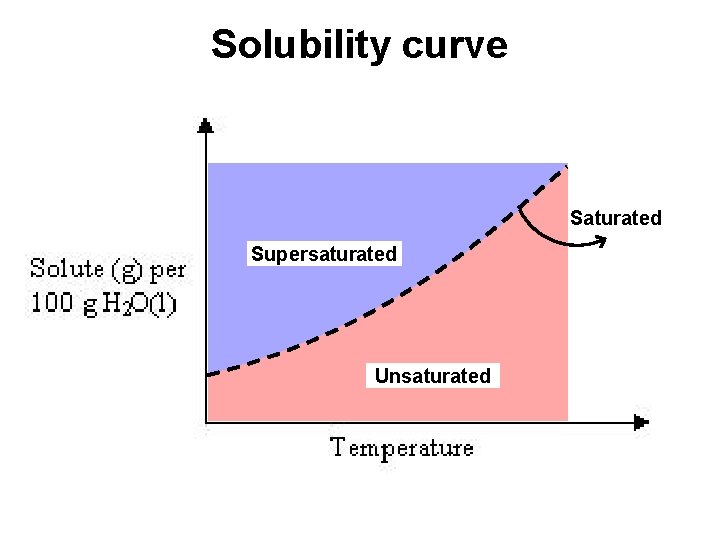
Solubility curve Saturated Supersaturated Unsaturated

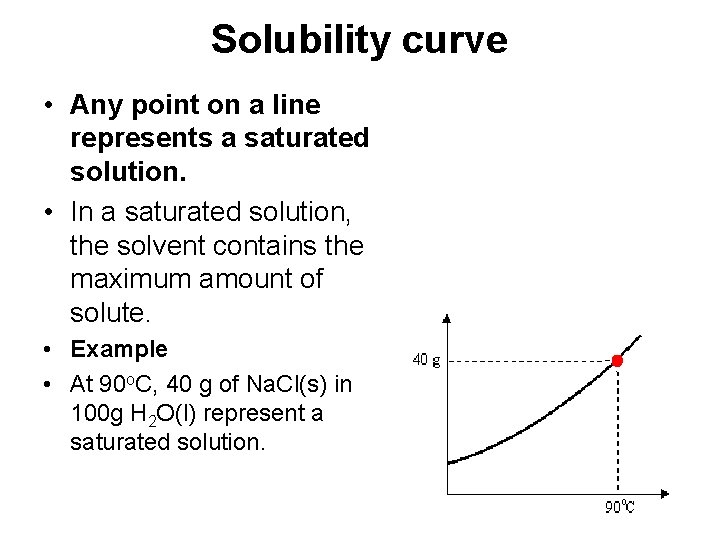
Solubility curve • Any point on a line represents a saturated solution. • In a saturated solution, the solvent contains the maximum amount of solute. • Example • At 90 o. C, 40 g of Na. Cl(s) in 100 g H 2 O(l) represent a saturated solution.

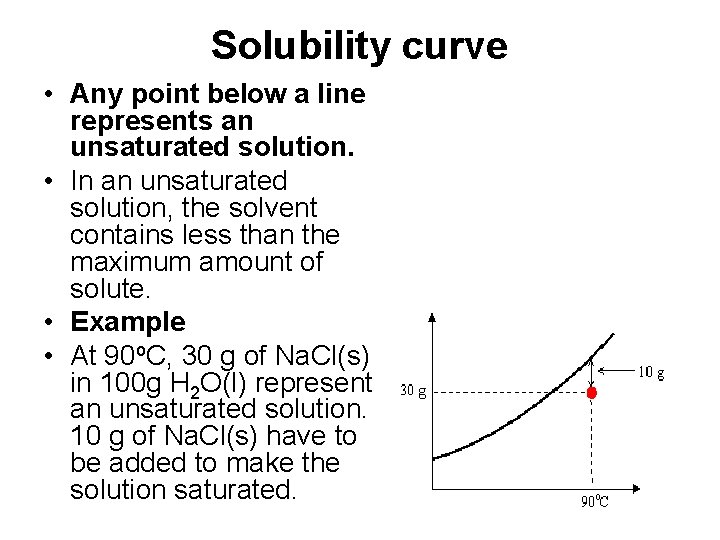
Solubility curve • Any point below a line represents an unsaturated solution. • In an unsaturated solution, the solvent contains less than the maximum amount of solute. • Example • At 90 o. C, 30 g of Na. Cl(s) in 100 g H 2 O(l) represent an unsaturated solution. 10 g of Na. Cl(s) have to be added to make the solution saturated.

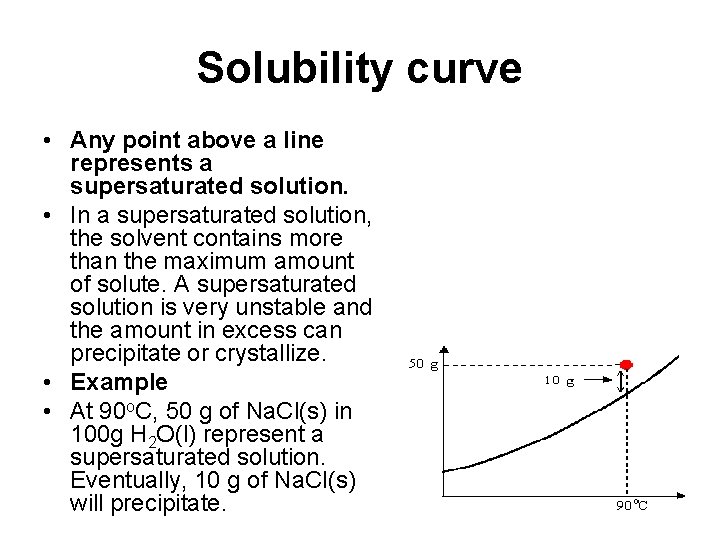
Solubility curve • Any point above a line represents a supersaturated solution. • In a supersaturated solution, the solvent contains more than the maximum amount of solute. A supersaturated solution is very unstable and the amount in excess can precipitate or crystallize. • Example • At 90 o. C, 50 g of Na. Cl(s) in 100 g H 2 O(l) represent a supersaturated solution. Eventually, 10 g of Na. Cl(s) will precipitate.


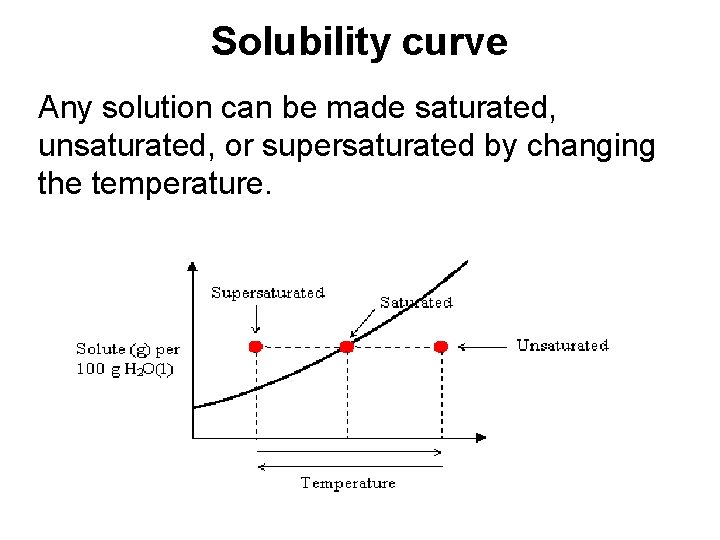
Solubility curve Any solution can be made saturated, unsaturated, or supersaturated by changing the temperature.

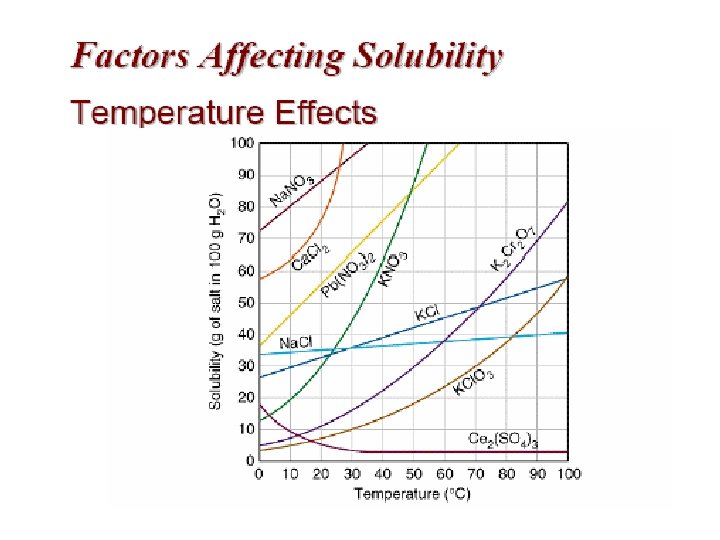
SOLUBILITY The solubility of a solute in a given amount of solvent is dependent on the temperature, the pressure, and the chemical natures of the solute and solvent.

Temperature • In general, as the temperature of a solution increases the solubility increases. • Increasing the solution temperature allows more sugar to go into solution. Therefore, it is an endothermic process (heat is on the reactant side).

Effect of Temperature on Solubility • The solubility of solutes is dependent on temperature. When a solid dissolves in a liquid, a change in the physical state of the solid analogous to melting takes place. Heat is required to break the bonds holding the molecules in the solid together. • At the same time, heat is given off during the formation of new solute -- solvent bonds.

Increase in solubility with temperature • If the heat given off in the hydration process is less than the heat required to break apart the solid, the net dissolving reaction is endothermic (energy required). The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids. • The use of first-aid instant cold packs is an application of this solubility principle. A salt such as ammonium nitrate is dissolved in water after a sharp blow breaks the containers for each. The dissolving reaction is endothermic - requires heat. Therefore the heat is drawn from the surroundings, the pack feels cold.

Molecules in a cold liquid are moving relatively slowly and therefore do not have much energy.

Molecules in a hot liquid are moving fast. Fast moving molecules have a significant kinetic energy.



Decrease in solubility with temperature • If the heat given off in the hydration process is greater than the heat required to break apart the solid, the net dissolving reaction is exothermic (energy given off). • The addition of more heat (increases temperature) inhibits the dissolving reaction since excess heat is already being produced by the reaction. This situation is not very common where an increase in temperature produces a decrease in solubility.

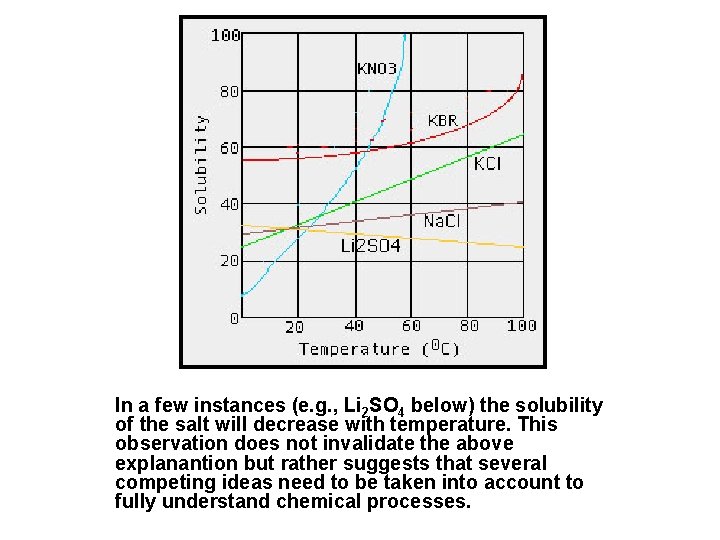
In a few instances (e. g. , Li 2 SO 4 below) the solubility of the salt will decrease with temperature. This observation does not invalidate the above explanantion but rather suggests that several competing ideas need to be taken into account to fully understand chemical processes.

Solubility of Gases vs. Temperature • The variation of solubility for a gas with temperature can be determined by examining the graph below: • As the temperature increases, the solubility of a gas decrease as shown by the downward trend in the graph.

Solubility of Gases vs. Temperature • More gas is present in a solution with a lower temperature compared to a solution with a higher temperature. • The reason for this gas solubility relationship with temperature is very similar to the reason that vapor pressure increases with temperature. Increased temperature causes an increase in kinetic energy. The higher kinetic energy causes more motion in molecules which break intermolecular bonds and escape from solution. • This gas solubility relationship can be remembered if you think about what happens to a "soda pop" as it stands around for awhile at room temperature. The taste is very "flat" since more of the "tangy" carbon dioxide bubbles have escaped. Boiled water also tastes "flat" because all of the oxygen gas has been removed by heating.

Summary of temperature effect on solubility • solid in liquid: • solubility of an endothermic dissolving solid in a liquid increases with increasing temperature, • but for an exothermic dissolving one solubility decreases with increasing temperature. • liquid in liquid: • for partially dissolving liquids like dimethyl ether(CH 3 -OCH 3 ) in water (H 2 O), solubilty increases with increasing temperature, • but for a completely dissolving liquids like ethyl alcohol( C 2 H 5 OH) in water( H 2 O) , solubility decreases with increasing temperature. • gas in liquid: • solubility of a gas in a liquid almost always decreases with increasing temperature

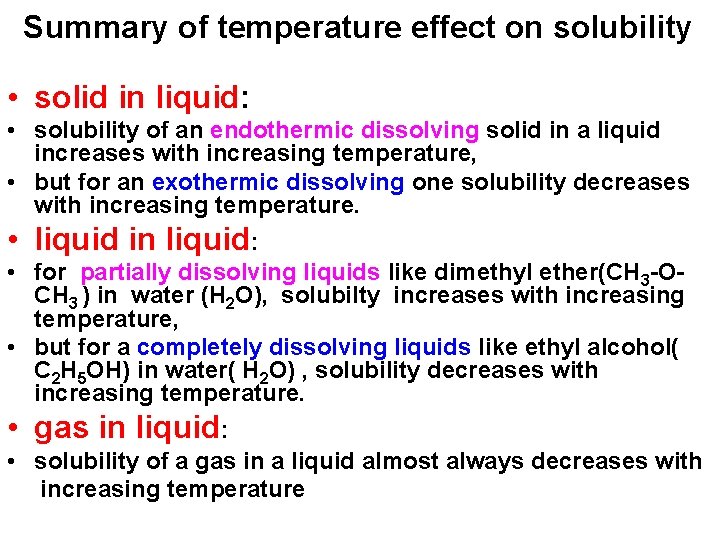
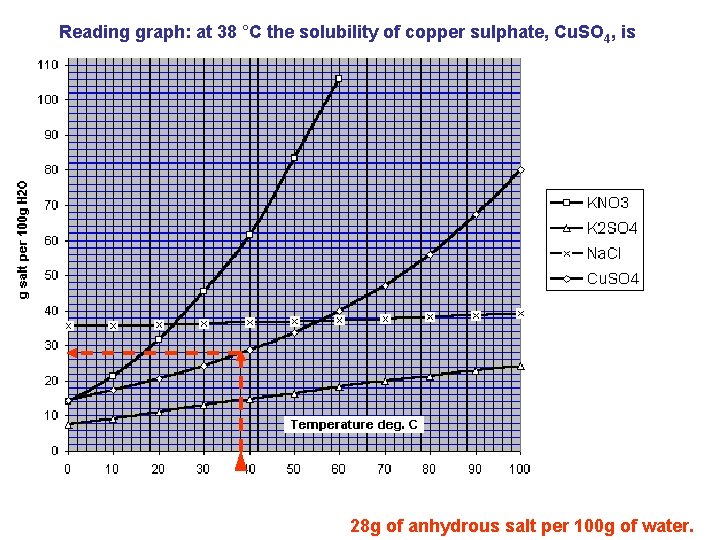
Reading graph: at 38 °C the solubility of copper sulphate, Cu. SO 4, is 28 g of anhydrous salt per 100 g of water.

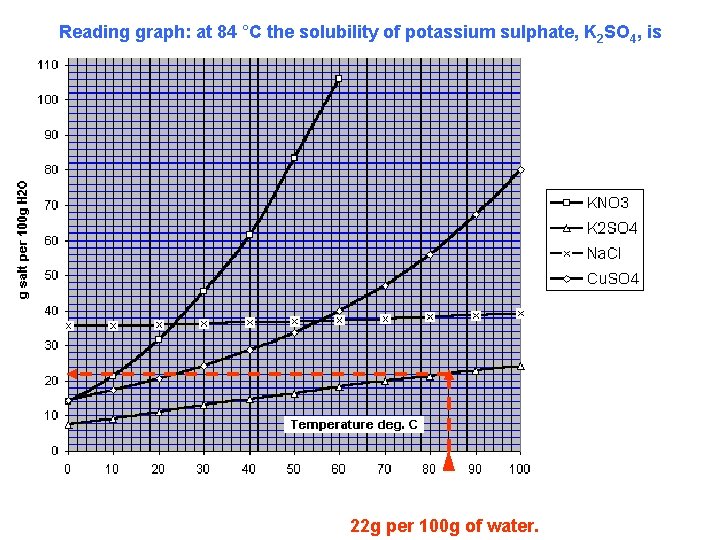
Reading graph: at 84 °C the solubility of potassium sulphate, K 2 SO 4, is 22 g per 100 g of water.

Ex Q 1: How much potassium nitrate will dissolve in 20 g of water at 34 °C? At 34 °C the solubility is 52 g per 100 g of water, so scaling down, 52 x 20 / 100 = 10. 4 g will dissolve in 20 g of water.

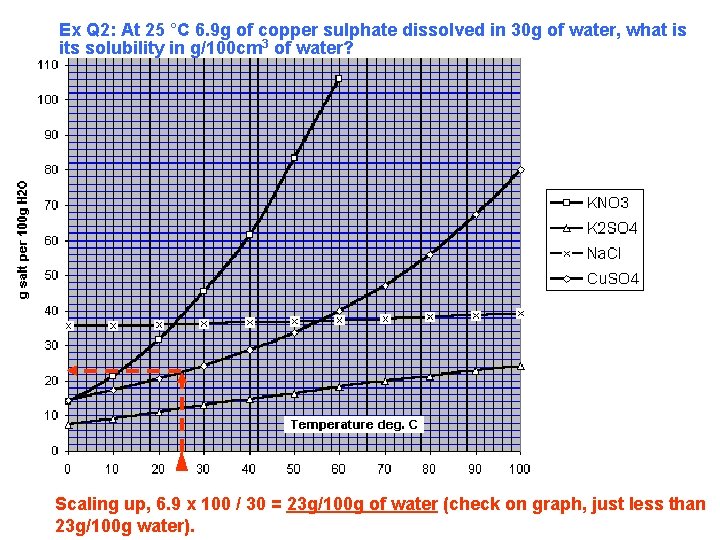
Ex Q 2: At 25 °C 6. 9 g of copper sulphate dissolved in 30 g of water, what is its solubility in g/100 cm 3 of water? Scaling up, 6. 9 x 100 / 30 = 23 g/100 g of water (check on graph, just less than 23 g/100 g water).

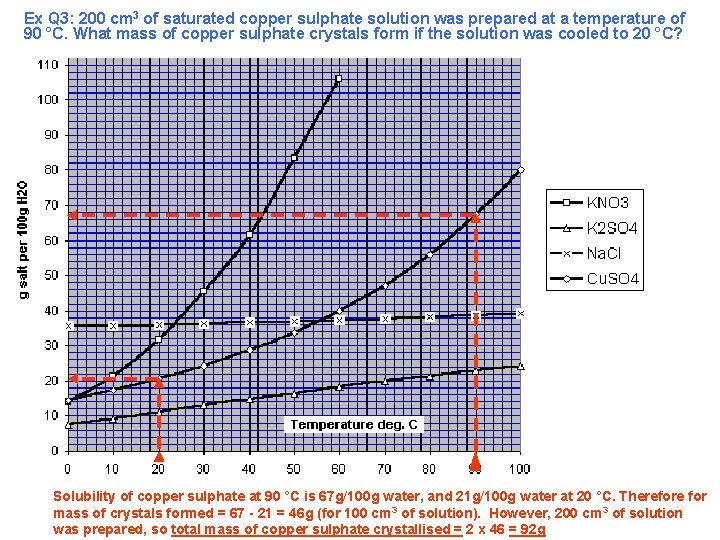
Ex Q 3: 200 cm 3 of saturated copper sulphate solution was prepared at a temperature of 90 °C. What mass of copper sulphate crystals form if the solution was cooled to 20 °C? Solubility of copper sulphate at 90 °C is 67 g/100 g water, and 21 g/100 g water at 20 °C. Therefore for mass of crystals formed = 67 - 21 = 46 g (for 100 cm 3 of solution). However, 200 cm 3 of solution was prepared, so total mass of copper sulphate crystallised = 2 x 46 = 92 g

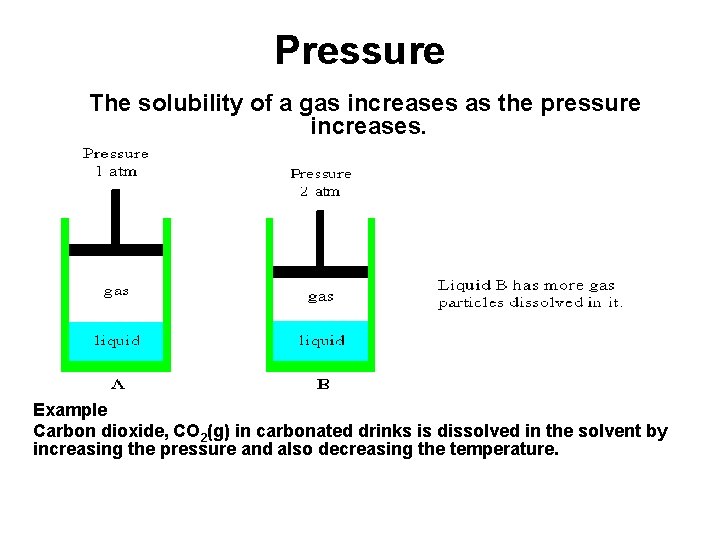
Pressure The solubility of a gas increases as the pressure increases. Example Carbon dioxide, CO 2(g) in carbonated drinks is dissolved in the solvent by increasing the pressure and also decreasing the temperature.

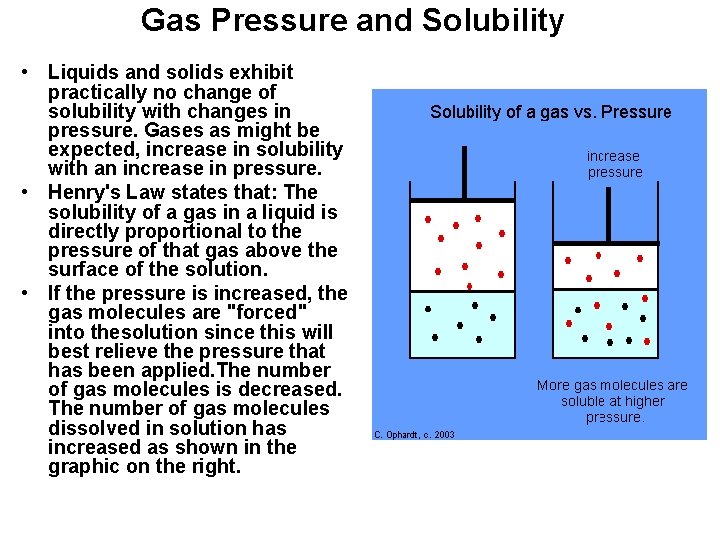
Gas Pressure and Solubility • Liquids and solids exhibit practically no change of solubility with changes in pressure. Gases as might be expected, increase in solubility with an increase in pressure. • Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution. • If the pressure is increased, the gas molecules are "forced" into thesolution since this will best relieve the pressure that has been applied. The number of gas molecules is decreased. The number of gas molecules dissolved in solution has increased as shown in the graphic on the right.

Gas Pressure and Solubility • Carbonated beverages provide the best example of this phenomena. All carbonated beverages are bottled under pressure to increase the carbon dioxide dissolved in solution. • When the bottle is opened, the pressure above the solution decreases. As a result, the solution effervesces and some of the carbon dioxide bubbles off. • Quiz: Champagne continues to ferment in the bottle. The fermentation produces CO 2. Why is the cork wired on a bottle of champagne? • Answer: As more CO 2 is formed , the pressure of the gas increase. The wire is to prevent the cork from blowing off.

Gas Pressure and Solubility • Deep sea divers may experience a condition called the "bends" if they do not readjust slowly to the lower pressure at the surface. • As a result of breathing compressed air and being subjected to high pressures caused by water depth, the amount of nitrogen dissolved in blood and other tissues increases. • If the diver returns to the surface too rapidly, the nitrogen forms bubbles in the blood as it becomes less soluble due to a decrease in pressure. The nitrogen bubbles can cause great pain and possibly death. • To alleviate this problem somewhat, artificial breathing mixtures of oxygen and helium are used. Helium is only one-fifth as soluble in blood as nitrogen. As a result, there is less dissolved gas to form bubbles.

Gas Pressure and Solubility • Quiz: If a diver had the "bends", describe how this can be treated. • Answer: Decompression chambers are used to keep a high pressure and gradually lower the pressure. • Another application of Henry's Law is in the administration of anesthetic gases. If the partial pressure of the anesthetic gas is increased, the anesthetic solubility increases in the blood.

Gas Pressure and Solubility • Quiz: The amount of dissolved oxygen in a mountain lake at 10, 000 ft and 50 o. F is __? _ than the amount of dissolved oxygen in a lake near sea level at 50 o. F. • Answer: Less at higher altitude because less pressure. • A Coke at room temperature will have __? _ carbon dioxide in the gas space above the liquid than an ice cold bottle. • Answer: More gas, because the warm coke can hold less of the gas in solution.

Gas Pressure and Solubility • Hyperbaric therapy, which involves exposure to oxygen at higher than atmospheric pressure may be used to treat hypoxia (low oxygen supply in the tissues). Explain how the treatment works. • Answer: The increase in pressure in the chamber will cause more gases to enter into lungs.

The rate of solution is a measure of how fast a substance dissolves. Some of the factors determining the rate of solution are: • size of the particles -- When a solute dissolves, the action takes place only at the surface of each particle. When the total surface area of the solute particles is increased, the solute dissolves more rapidly. Breaking a solute into smaller pieces increases its surface area and hence its rate of solution. (Sample problem: a cube with sides 1. 0 cm long is cut in half, producing two pieces with dimensions of 1. 0 cm x 0. 50 cm. How much greater than the surface area of the original cube is the combined surface areas of the two pieces? • 2. 0 cm 2 • stirring -- With liquid and solid solutes, stirring brings fresh portions of the solvent in contact with the solute, thereby increasing the rate of solution.

The rate of solution • amount of solute already dissolved -- When there is little solute already in solution, dissolving takes place relatively rapidly. As the solution approaches the point where no solute can be dissolved, dissolving takes place more slowly. • temperature -- For solid, liquid and gaseous solutes, changing the temperature not only changes the amount of solute that will dissolve but also changes the rate at which the solute will dissolve.

How do I get sugar to dissolve faster in my iced tea? Stir, and stir Fresh solvent contact and interaction with solute Add sugar to warm tea then add ice Faster rate of dissolution at higher temperature Grind the sugar to a powder Greater surface area, more solute-solvent interaction

Chemical natures of the solute and solvent A polar solute will dissolve in a polar solvent but not in a nonpolar solvent. The adage "like dissolves like" is very useful. Example Alcohol (polar substance) dissolves in water (polar substance) Water (polar substance) does not dissolve in oil (nonpolar substance)

Nature of the solute and solvent “Likes dissolve likes” When two similar liquids - here water and methanol- are mixed, the molecules are intermingled. The mixture has a more disorderly arrangement of molecules than the separate liquids. It is this disordering process that largely drives solution formation. • polar solute/polar solvent: • ethanol, salt, sugar in water • nonpolar solute/nonpolar solvent: • Iodine in carbontetrachloride, gasoline or benzene

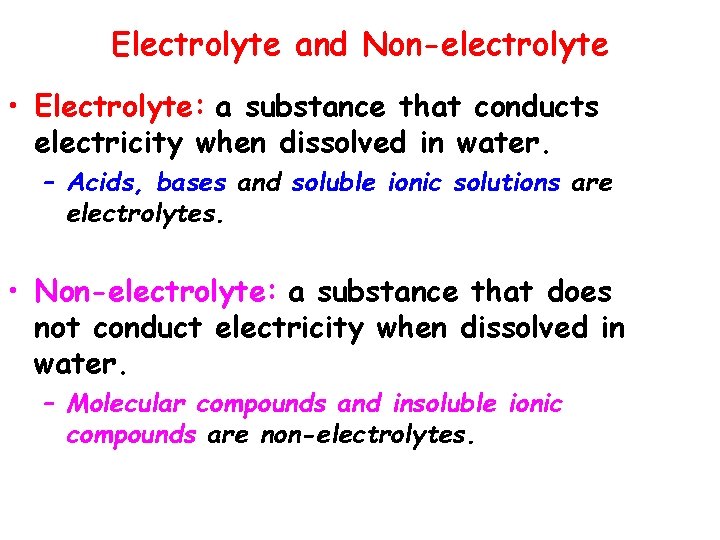
Electrolyte and Non-electrolyte • Electrolyte: a substance that conducts electricity when dissolved in water. – Acids, bases and soluble ionic solutions are electrolytes. • Non-electrolyte: a substance that does not conduct electricity when dissolved in water. – Molecular compounds and insoluble ionic compounds are non-electrolytes.

Electrolytes • Some solutes can dissociate into ions. • Electric charge can be carried.

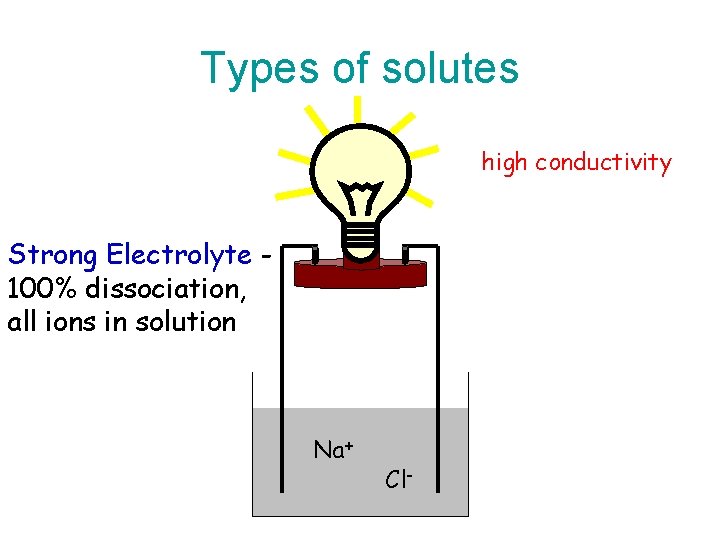
Types of solutes high conductivity Strong Electrolyte 100% dissociation, all ions in solution Na+ Cl-

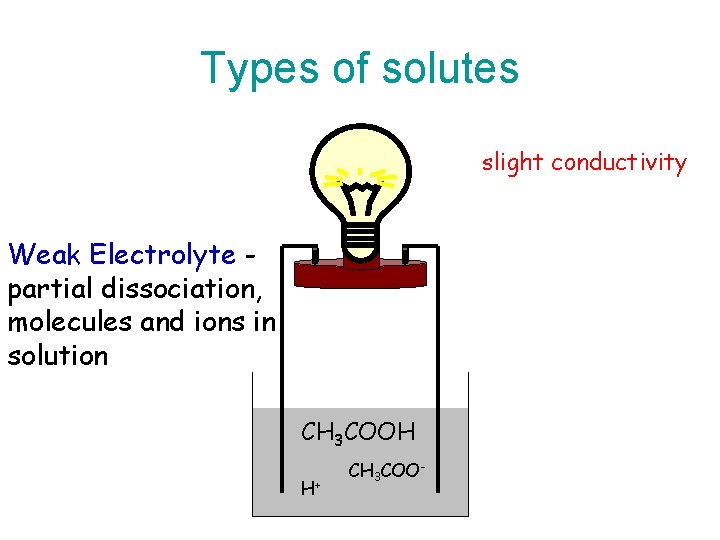
Types of solutes slight conductivity Weak Electrolyte partial dissociation, molecules and ions in solution CH 3 COOH H+ CH 3 COO-

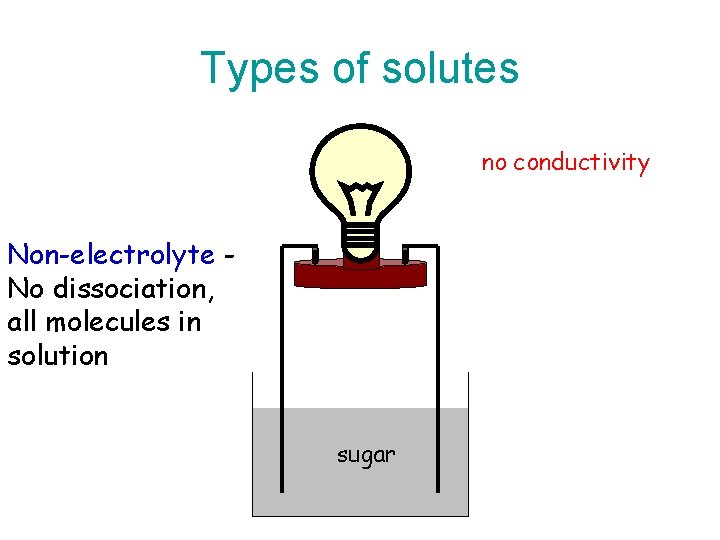
Types of solutes no conductivity Non-electrolyte No dissociation, all molecules in solution sugar

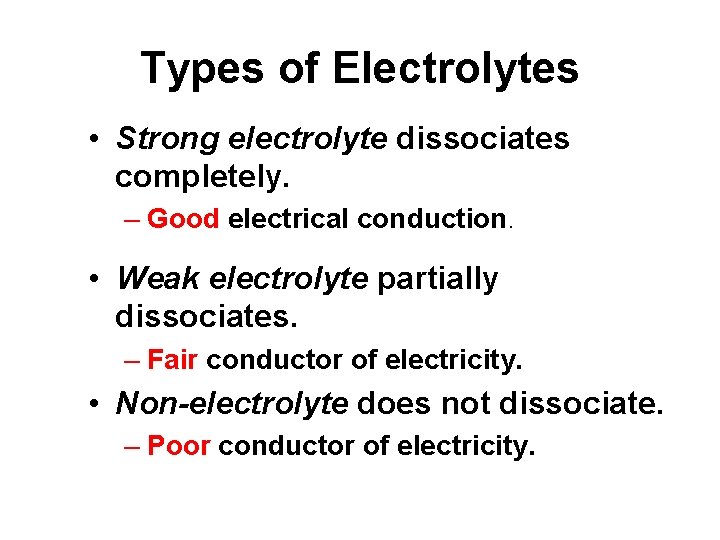
Types of Electrolytes • Strong electrolyte dissociates completely. – Good electrical conduction. • Weak electrolyte partially dissociates. – Fair conductor of electricity. • Non-electrolyte does not dissociate. – Poor conductor of electricity.

Representation of Electrolytes using Chemical Equations A strong electrolyte: Mg. Cl 2(s) → Mg 2+(aq) + 2 Cl- (aq) A weak electrolyte: -(aq) +H+(aq) CH 3 COOH(aq) → CH COO 3 ← A non-electrolyte: CH 3 OH(aq)

Strong Electrolytes Strong acids: HNO 3, H 2 SO 4, HCl. O 4 Strong bases: MOH (M = Na, K, Cs, Rb etc) Salts: All salts dissolving in water are completely ionized. Stoichiometry & concentration relationship Na. Cl (s) Na+ (aq) + Cl– (aq) Ca(OH)2 (s) Ca 2+(aq) + 2 OH– (aq) Al. Cl 3 (s) Al 3+ (aq) + 3 Cl– (aq) (NH 4)2 SO 4 (s) 2 NH 4 + (aq) +

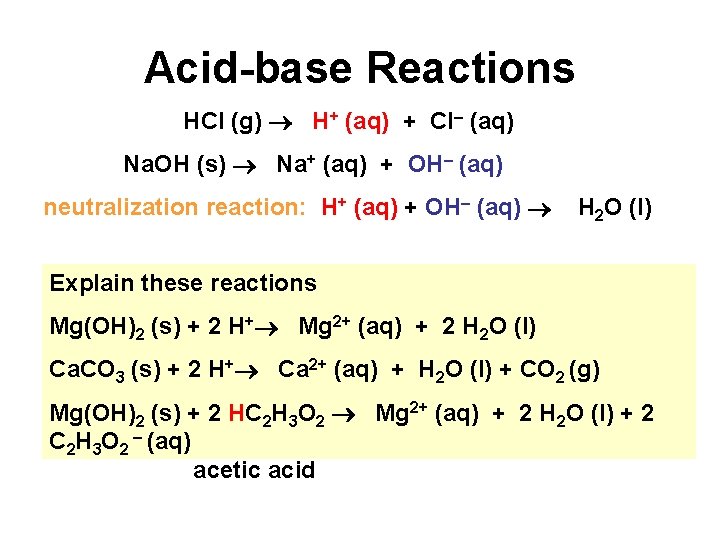
Acid-base Reactions HCl (g) H+ (aq) + Cl– (aq) Na. OH (s) Na+ (aq) + OH– (aq) neutralization reaction: H+ (aq) + OH– (aq) H 2 O (l) Explain these reactions Mg(OH)2 (s) + 2 H+ Mg 2+ (aq) + 2 H 2 O (l) Ca. CO 3 (s) + 2 H+ Ca 2+ (aq) + H 2 O (l) + CO 2 (g) Mg(OH)2 (s) + 2 HC 2 H 3 O 2 Mg 2+ (aq) + 2 H 2 O (l) + 2 C 2 H 3 O 2 – (aq) acetic acid

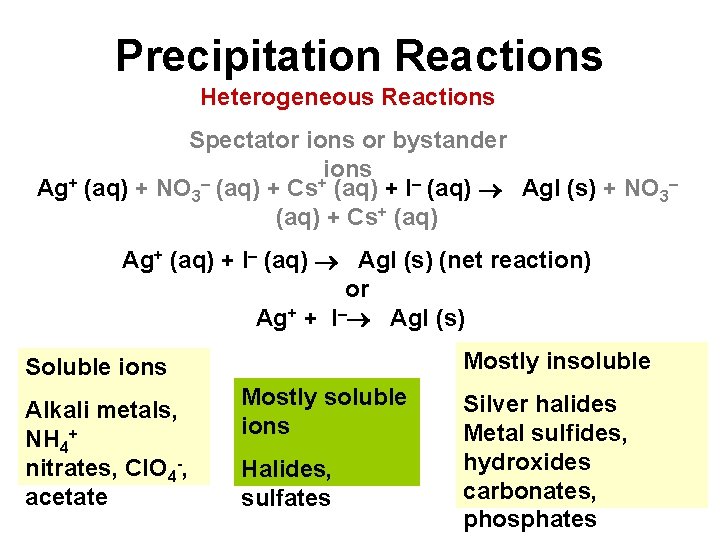
Precipitation Reactions Heterogeneous Reactions Spectator ions or bystander ions + – + Ag (aq) + NO 3 (aq) + Cs (aq) + I– (aq) Ag. I (s) + NO 3– (aq) + Cs+ (aq) Ag+ (aq) + I– (aq) Ag. I (s) (net reaction) or Ag+ + I– Ag. I (s) Mostly insoluble Soluble ions Alkali metals, NH 4+ nitrates, Cl. O 4 -, acetate Mostly soluble ions Halides, sulfates Silver halides Metal sulfides, hydroxides carbonates, phosphates

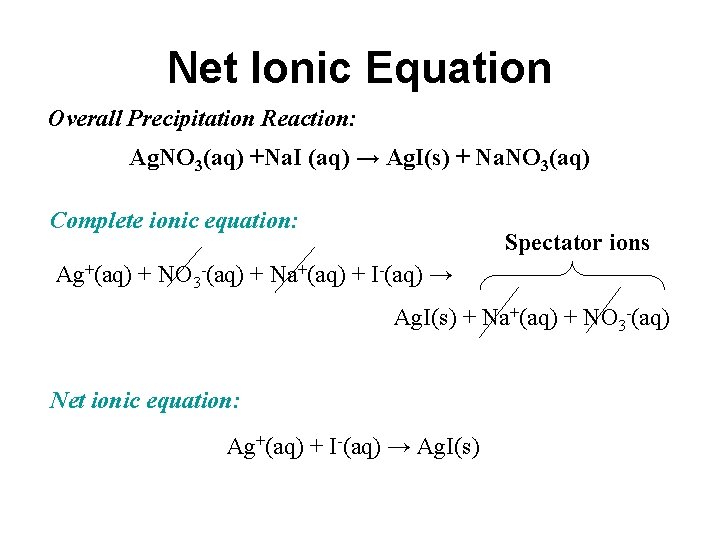
Net Ionic Equation Overall Precipitation Reaction: Ag. NO 3(aq) +Na. I (aq) → Ag. I(s) + Na. NO 3(aq) Complete ionic equation: Spectator ions Ag+(aq) + NO 3 -(aq) + Na+(aq) + I-(aq) → Ag. I(s) + Na+(aq) + NO 3 -(aq) Net ionic equation: Ag+(aq) + I-(aq) → Ag. I(s)

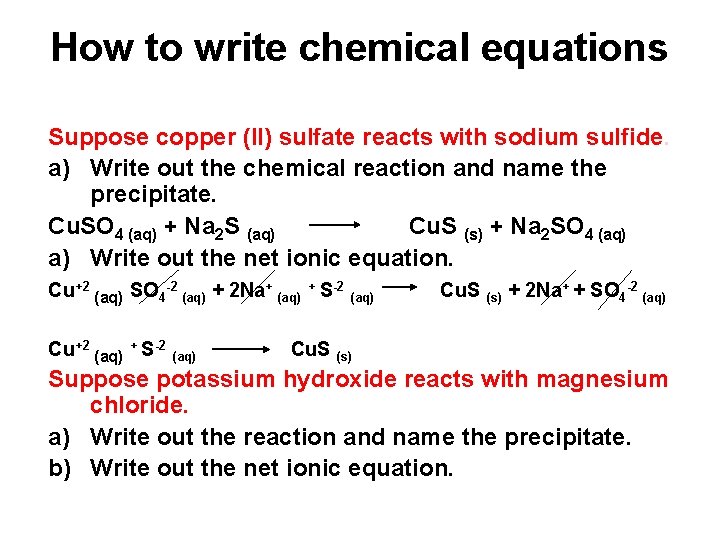
How to write chemical equations Suppose copper (II) sulfate reacts with sodium sulfide. a) Write out the chemical reaction and name the precipitate. Cu. SO 4 (aq) + Na 2 S (aq) Cu. S (s) + Na 2 SO 4 (aq) a) Write out the net ionic equation. Cu+2 (aq) SO 4 -2 (aq) + 2 Na+ (aq) + S-2 (aq) Cu. S (s) + 2 Na+ + SO 4 -2 (aq) Cu+2 (aq) + S-2 (aq) Cu. S (s) Suppose potassium hydroxide reacts with magnesium chloride. a) Write out the reaction and name the precipitate. b) Write out the net ionic equation.


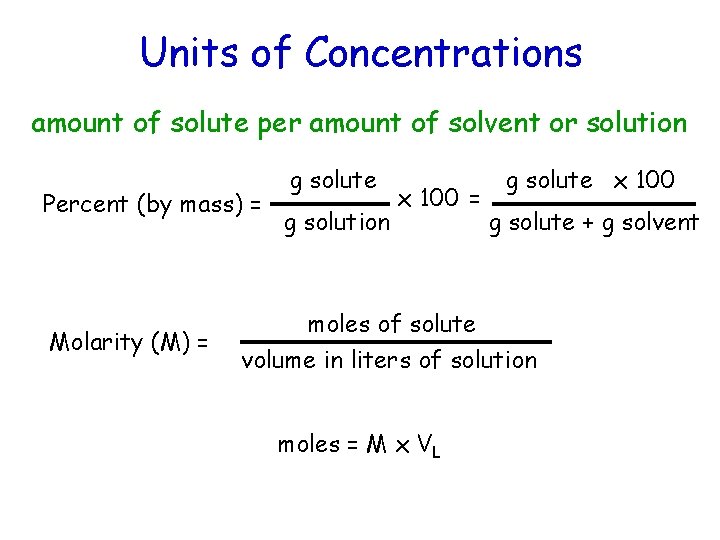
Units of Concentrations amount of solute per amount of solvent or solution Percent (by mass) = Molarity (M) = g solute g solution x 100 = g solute x 100 g solute + g solvent moles of solute volume in liters of solution moles = M x VL


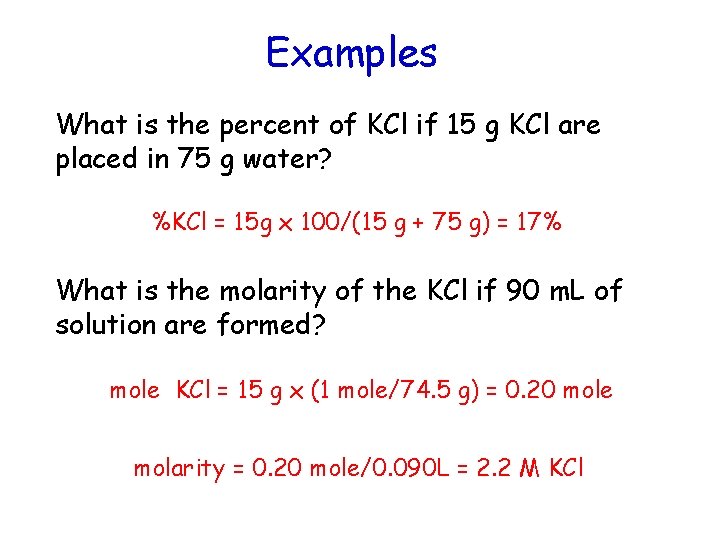
Examples What is the percent of KCl if 15 g KCl are placed in 75 g water? %KCl = 15 g x 100/(15 g + 75 g) = 17% What is the molarity of the KCl if 90 m. L of solution are formed? mole KCl = 15 g x (1 mole/74. 5 g) = 0. 20 mole molarity = 0. 20 mole/0. 090 L = 2. 2 M KCl

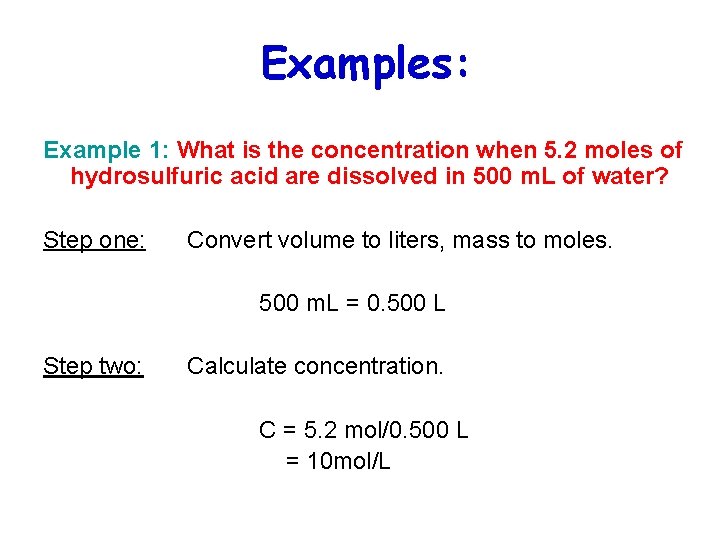
Examples: Example 1: What is the concentration when 5. 2 moles of hydrosulfuric acid are dissolved in 500 m. L of water? Step one: Convert volume to liters, mass to moles. 500 m. L = 0. 500 L Step two: Calculate concentration. C = 5. 2 mol/0. 500 L = 10 mol/L

• Example 2: What is the volume when 9. 0 moles are present in 5. 6 mol/L hydrochloric acid? • Example 3: How many moles are present in 450 m. L of 1. 5 mol/L calcium hydroxide? • Example 4: What is the concentration of 5. 6 g of magnesium hydroxide dissolved in 550 m. L? • Example 5: What is the volume of a 0. 100 mol/L solution that contains 5. 0 g of sodium chloride?

How many Tums tablets, each 500 mg Ca. CO 3, would it take to neutralize a quart of vinegar, 0. 83 M acetic acid (CH 3 COOH)? 2 CH 3 COOH(aq) + Ca. CO 3(s) Ca(CH 3 COO)2(aq) + H 2 O + CO 2(g) a quart moles acetic acid = 0. 83 moles/L x 0. 95 L = 0. 79 moles AA the mole ratio mole Ca. CO 3 = 0. 79 moles AA x (1 mole Ca. CO 3/2 moles AA) = 0. 39 moles Ca. CO 3 molar mass Ca. CO 3 = 0. 39 moles x 100 g/mole = 39 g Ca. CO 3 number of tablets = 39 g x (1 tablet/0. 500 g) = 79 tablets
