Solutions Mixtures with a solute and a solvent







- Slides: 13

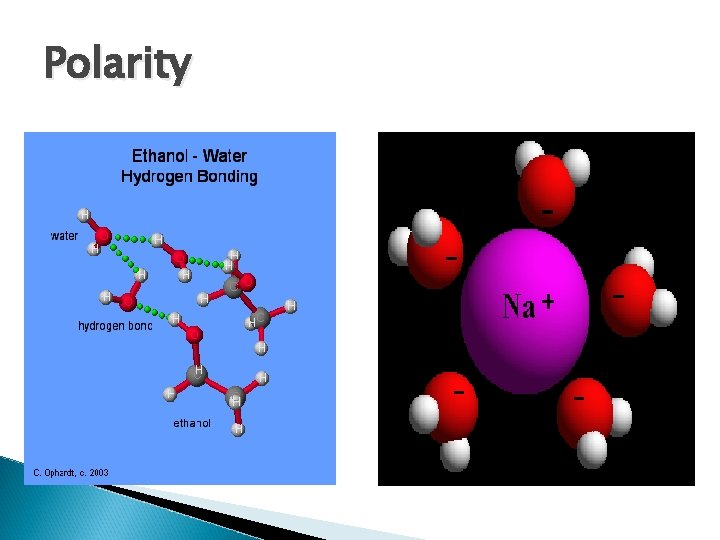
Solutions Mixtures with a solute and a solvent. ◦ Solute: the solute is what is being dissolved. ◦ Solvent: the solvent is what is doing the dissolving. How things dissolve… ◦ “Polar Molecules” are like magnets, one end is positive the other is negative. ◦ Polar molecules can only dissolve other polar molecules. ◦ Non-polar molecules must be dissolved by other non-polar molecules.

Polarity

Types of Solutions may exist as a gas, liquid, or solid depending on the state of matter of the solvent. ◦ Gas solutions Example – Air Solvent: Nitrogen Solute: Oxygen Carbonated Water Solvent: Water Solute: Carbon dioxide Vinegar Solvent: Water Solute: Acetic Acid ◦ Liquid Solutions Gas in Liquid Solid in Liquid Ocean Water Solvent: Water ◦ Solid Solutions Example – Steel Solute: Na. Cl Solvent: Iron Solute: Carbon

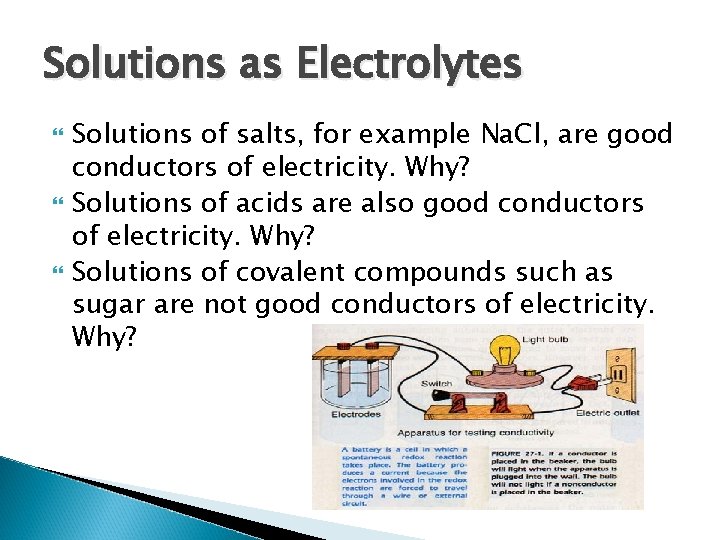
Solutions as Electrolytes Solutions of salts, for example Na. Cl, are good conductors of electricity. Why? Solutions of acids are also good conductors of electricity. Why? Solutions of covalent compounds such as sugar are not good conductors of electricity. Why?

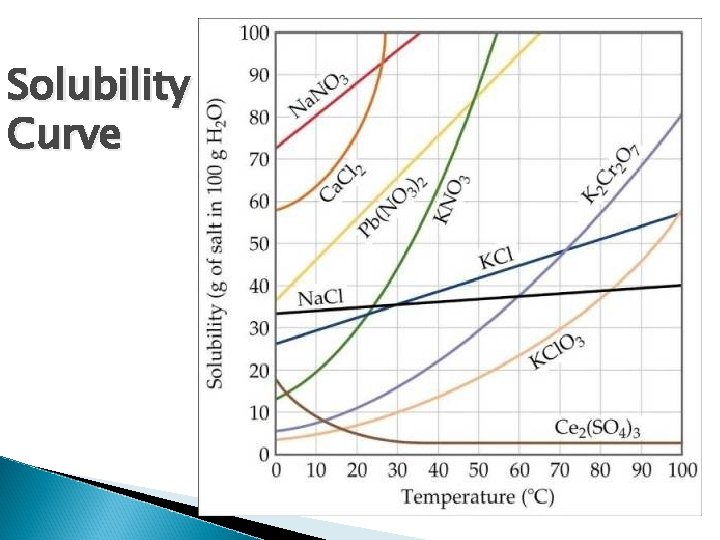
Solubility Refers to the maximum amount of solute that will dissolve in a given amount of solvent; at a specified temperature and pressure. Solubility can usually be expressed in grams of solute per 100 grams of solvent. ◦ Na. Cl (at 0 degrees Celsius) 35. 7 g/100 g H 2 O (at 20 degrees Celsius) 35. 9 g/100 g H 2 O (at 40 degrees Celsius ) 36. 4 g/100 g H 2 O

Factors that Effect Solubility Temperature – many substances are more soluble at higher temperatures. Pressure – solubility increases as a substances external pressure increases. Surface Area – increasing surface area increases solubility. Henry’s Law (for gases only): States that at a given temperature, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P) of the gas.

Solubility Curve

Solubility Cont. Saturated Solution – a solution that contains the maximum amount of dissolved solute for a given amount of solvent. Unsaturated Solution – contains a less dissolved solute for a given temperature and pressure than a saturated solution. Supersaturated Solution – a solution that has more solute dissolved than it can hold.

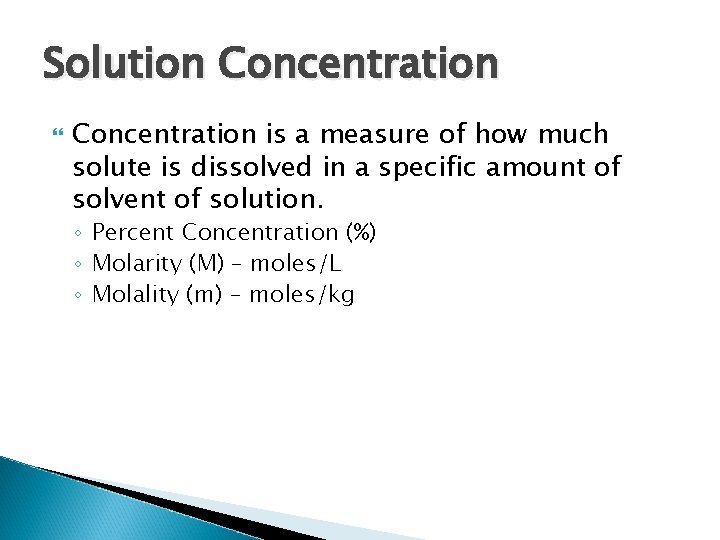
Solution Concentration is a measure of how much solute is dissolved in a specific amount of solvent of solution. ◦ Percent Concentration (%) ◦ Molarity (M) – moles/L ◦ Molality (m) – moles/kg

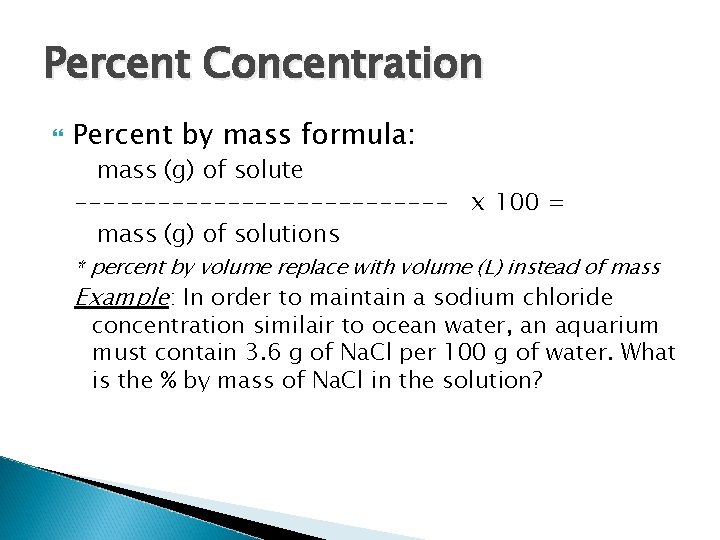
Percent Concentration Percent by mass formula: mass (g) of solute -------------- x 100 = mass (g) of solutions * percent by volume replace with volume (L) instead of mass Example: In order to maintain a sodium chloride concentration similair to ocean water, an aquarium must contain 3. 6 g of Na. Cl per 100 g of water. What is the % by mass of Na. Cl in the solution?

Molarity The number of moles of solute per liter (L) of solution. Molarity (M) Formula: moles of solute -------------liters of solution Example: What is the molarity of a solution produced when 4 moles of Na. Cl is dissolved by 1000 m. L of water?

Molality Sometimes we need to use mass because it does not change with temperature. Molality formula (m): moles of solute ------------kilograms of solvent

Diluting Solutions We use this formula in chemistry when we dilute solutions. Dilution Formula: M 1 V 1 = M 2 V 2 M = Molarity (M) V = Volume (L)