Welcome Please have a seat quietly After the












- Slides: 18

Welcome! – Please have a seat quietly. After the bell, we will watch a quick video about lesson 3. You may take notes if you wish; they may help with your storyboard – Take out your Water Cycle Story Board from the tray and begin working on it – You will have 15 minutes to finish

Notes • Take out your notebook • Grab your Clicker • Get ready to take some notes!


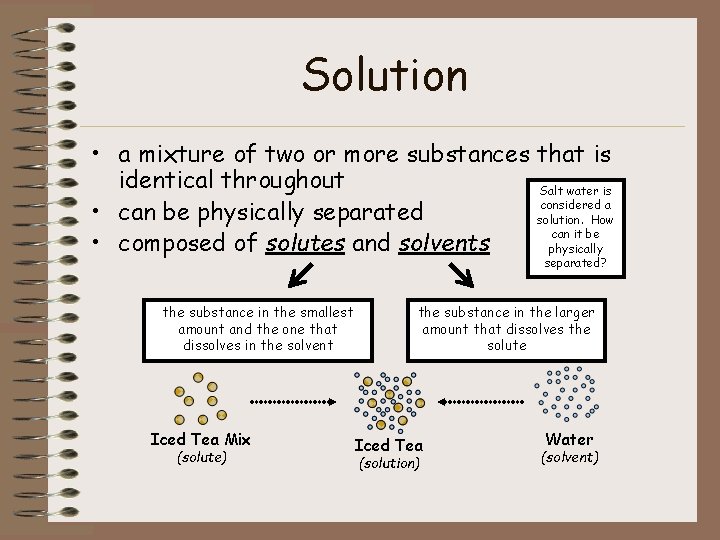
Solution • a mixture of two or more substances that is identical throughout Salt water is considered a • can be physically separated solution. How can it be • composed of solutes and solvents physically separated? the substance in the smallest amount and the one that dissolves in the solvent Iced Tea Mix (solute) the substance in the larger amount that dissolves the solute Iced Tea (solution) Water (solvent)

What is a solution? Solute/solvent Solute Solvent The solute is the substance that gets Dissolved. Dissolving The solvent does the ____.

How can you remember? § Think, Pair, Share l l Think for 1 minute about how you could remember the difference between solute and solvent. Pair up with your shoulder partner, 15 seconds each, share your strategy for memorization

This is the substance with the smaller amount? A. Solution B. Solute C. Solvent

Which of these best represents a solution? § A. § B. § C. A solution is a type of homogeneous mixture where the parts can be physically separated, and the parts are equally spread apart throughout the sample.

Solutions • Solutions are homogeneous mixtures of two or more pure substances. • In a solution, the solute is dispersed uniformly throughout the solvent. Solutions

What is a solution? Soluble/insoluble A substance that is Soluble can be dissolved.

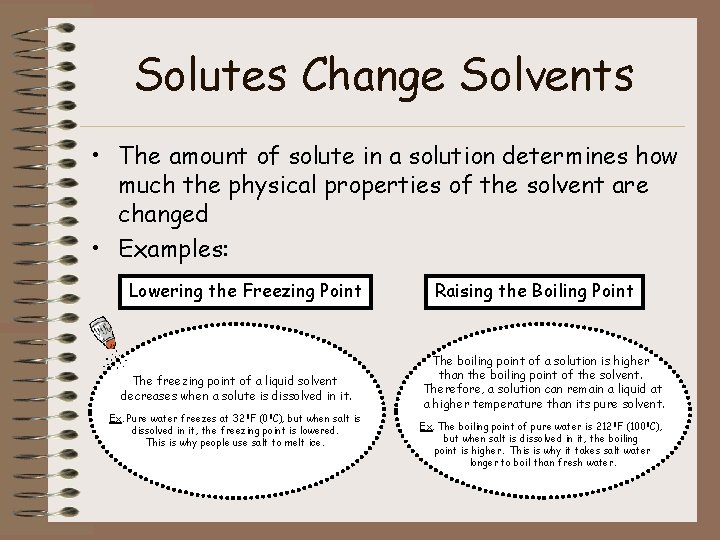
Solutes Change Solvents • The amount of solute in a solution determines how much the physical properties of the solvent are changed • Examples: Lowering the Freezing Point The freezing point of a liquid solvent decreases when a solute is dissolved in it. Ex. Pure water freezes at 32 0 F (00 C), but when salt is dissolved in it, the freezing point is lowered. This is why people use salt to melt ice. Raising the Boiling Point The boiling point of a solution is higher than the boiling point of the solvent. Therefore, a solution can remain a liquid at a higher temperature than its pure solvent. Ex. The boiling point of pure water is 2120 F (1000 C), but when salt is dissolved in it, the boiling point is higher. This is why it takes salt water longer to boil than fresh water.

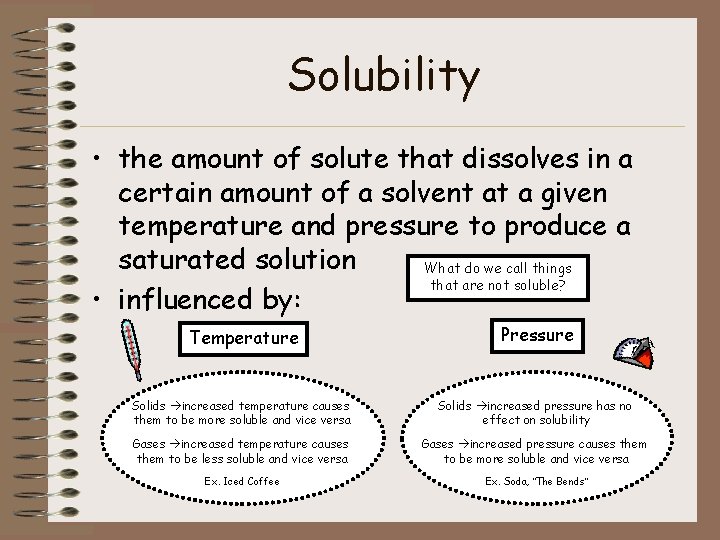
Solubility • the amount of solute that dissolves in a certain amount of a solvent at a given temperature and pressure to produce a saturated solution What do we call things that are not soluble? • influenced by: Temperature Pressure Solids increased temperature causes them to be more soluble and vice versa Solids increased pressure has no effect on solubility Gases increased temperature causes them to be less soluble and vice versa Gases increased pressure causes them to be more soluble and vice versa Ex. Iced Coffee Ex. Soda, “The Bends”

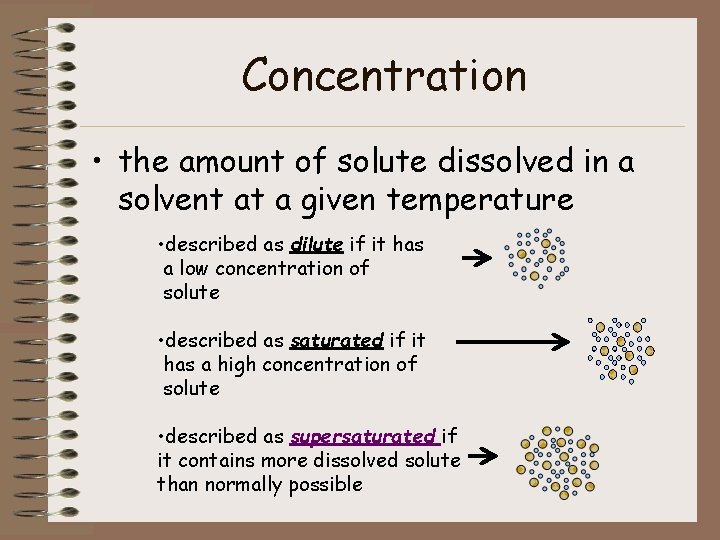
Concentration • the amount of solute dissolved in a solvent at a given temperature • described as dilute if it has a low concentration of solute • described as saturated if it has a high concentration of solute • described as supersaturated if it contains more dissolved solute than normally possible

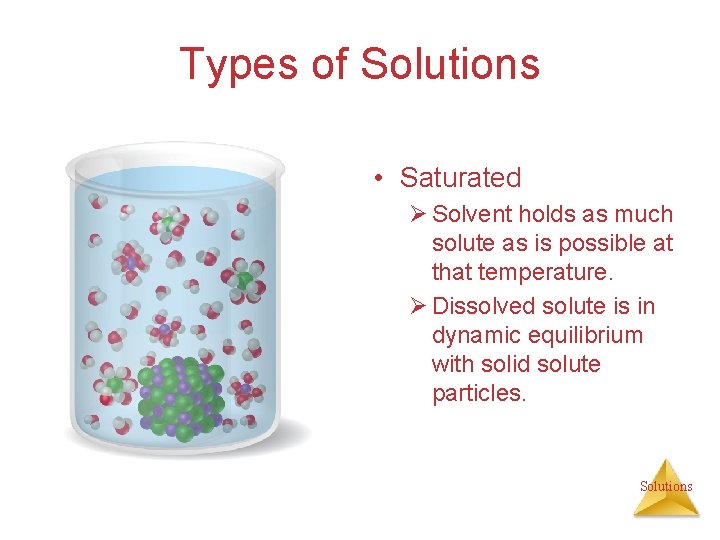
Types of Solutions • Saturated Ø Solvent holds as much solute as is possible at that temperature. Ø Dissolved solute is in dynamic equilibrium with solid solute particles. Solutions

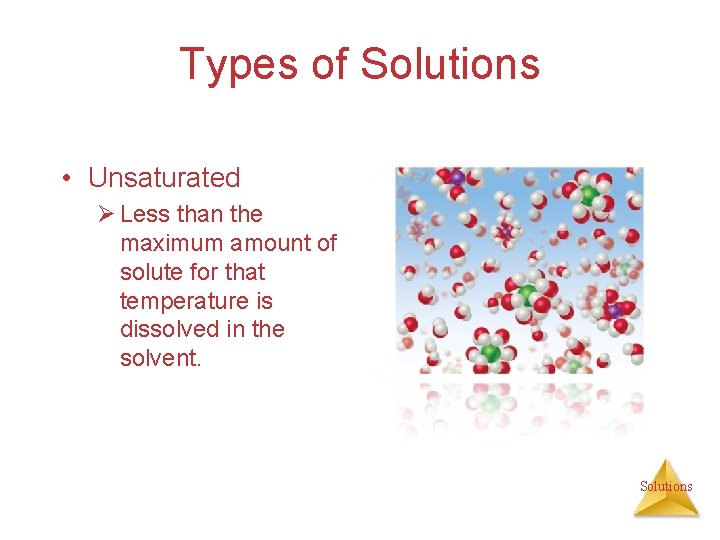
Types of Solutions • Unsaturated Ø Less than the maximum amount of solute for that temperature is dissolved in the solvent. Solutions

Types of Solutions • Supersaturated Ø Solvent holds more solute than is normally possible at that temperature. Ø These solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask. Solutions

Separating Mixtures Because they are formed physically, they can be separated in various ways: – DECANTING GOLD – DISTILLATION – FILTRATION – CHROMATOGRAPHY

Gestures/Pictionary • You and your partner are a pair. • You will take a card from the deck and act out/draw the word for the other group. • Each group will have 30 seconds to try to guess the correct term.
 Welcome please take a seat
Welcome please take a seat He took his seat quietly
He took his seat quietly Awubis
Awubis Il capitano
Il capitano Good morning please have a seat
Good morning please have a seat Good morning please have a seat
Good morning please have a seat Good afternoon please
Good afternoon please Good evening have a seat
Good evening have a seat Please sit down and your seat belts
Please sit down and your seat belts Please sit down and your seat belts
Please sit down and your seat belts Good morning please have a seat
Good morning please have a seat After me after me after me
After me after me after me John 14
John 14 Hey hey people
Hey hey people Please take a seat
Please take a seat Please be quiet. i (try) to sleep
Please be quiet. i (try) to sleep Please extend your warm welcome
Please extend your warm welcome Welcome please sign in
Welcome please sign in Raise your right hand and repeat after me
Raise your right hand and repeat after me