MATTER MATTER Matter MATTER What is matter Matter









- Slides: 11

MATTER MATTER Matter MATTER

What is matter?

Matter is anything that has mass and occupies space. All matter is made up of tiny particles, that are in constant motion. These particles can be atoms, molecules or ions. This idea is called the kinetic theory of matter. This theory is supported by Diffusion , Osmosis and Brownian motion. ( try and focus on how these explain that matter is made up of particles that are in constant motion)

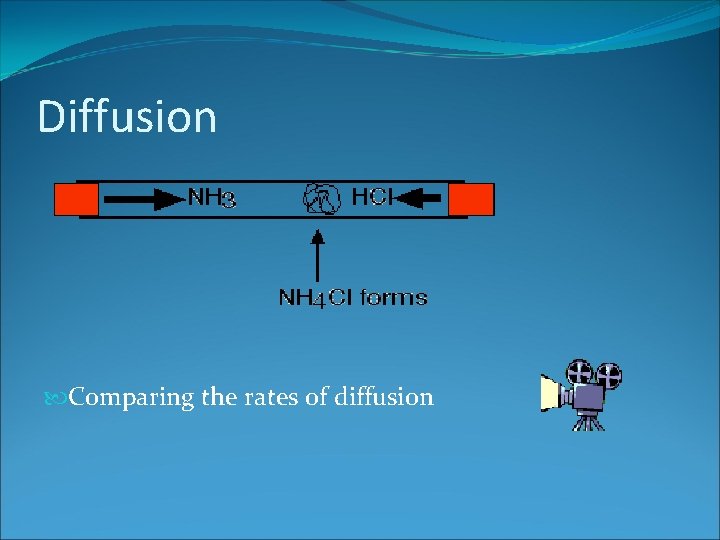
DIFFUSION What do you see happening? Explain what is occurring.

DIFFUSION Diffusion is the movement of particles from an area of high concentration to an area of low concentration, until the particles are evenly distributed. Examples: - Any smell (flower fragrance, garbage stink, body odour)- gas molecules diffuse into the air so you can smell it. Drop a drop of ink into a glass of water - the ink would diffuse

Diffusion Comparing the rates of diffusion

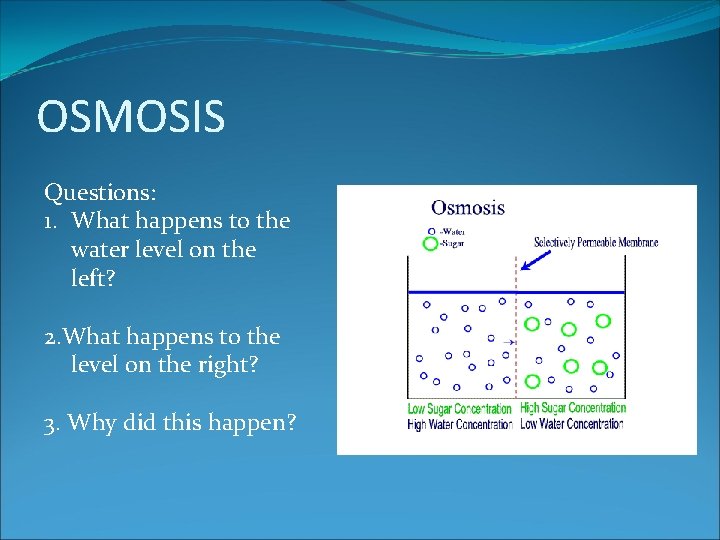
OSMOSIS Questions: 1. What happens to the water level on the left? 2. What happens to the level on the right? 3. Why did this happen?

Osmosis is the movement of water particles from an area of high water concentration to an area of low water concentration, through a semi-permeable membrane.

Examples of Osmosis If you put rice into a bowl of water, the water will move into the rice causing them to swell, while causing the water level to drop. When the stem of a plant is cut and placed in water (for example a vase), the water will move up through the stem by a process of osmosis, in which the water is flowing to the higher concentration of solvents (found in the plant).

Brownian Motion

Don’t Be Shy. . Explain the following: 1. Perfume sprayed in one corner of a room is soon detected in the entire room. 2. When a few drops of red colouring are placed in a beaker of water, after a short while, the entire liquid is red. 3. When a prune is placed in water, it swells
 Composition of matter section 1
Composition of matter section 1 Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers What is grey matter
What is grey matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Brain falx
Brain falx Flow of energy vs flow of matter
Flow of energy vs flow of matter Gray matter
Gray matter Principle over matter
Principle over matter All matter is made up of what
All matter is made up of what Uses of heat
Uses of heat





















