Review Notes 9 Matter What is Matter Matter






- Slides: 11

Review Notes #9 Matter

What is Matter? • Matter is anything that takes up space. • That space is called volume • Building blocks of matter are elements. • Smallest part of an element is an atom.

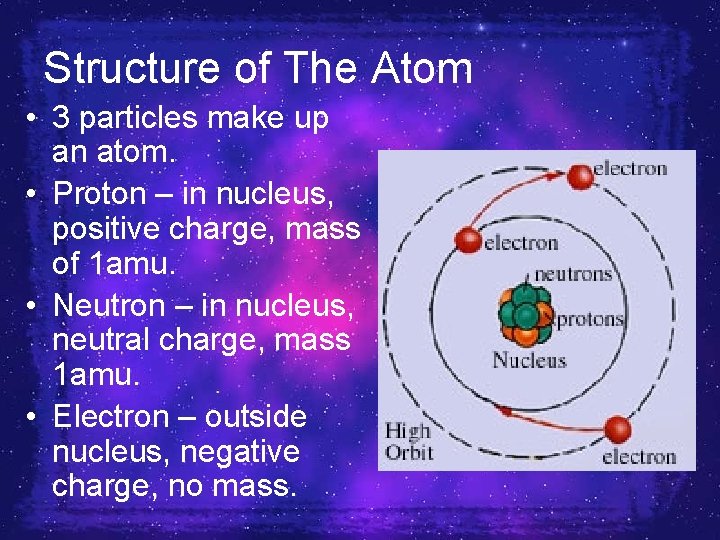
Structure of The Atom • 3 particles make up an atom. • Proton – in nucleus, positive charge, mass of 1 amu. • Neutron – in nucleus, neutral charge, mass 1 amu. • Electron – outside nucleus, negative charge, no mass.

Compounds and Periodic Table • A compound is formed from two or more elements. • Elements are arranged on the periodic table by atomic number • Sorted by characteristics.

Phases of Matter • Solid – particles are close together, slow moving, definite volume and shape • Liquid - Particles further apart and moving a little faster. Definite volume but not shape. • Gas - Particles very far apart and moving very fast. No definite shape or volume.

Phase Changes • Melting – Solid to Liquid, energy absorbed • Freezing – liquid to solid, energy released • Vaporization – liquid to gas, energy is absorbed. • Condensation – gas to liquid, energy is released • Water – boiling point 100 o. C, freezing point 0 o. C

Solutions • A mixture that stays evenly distributed like salt water. • Salt is soluble in water. Sand is insoluble in water • Things that cause a faster dissolving rate are, stirring, increase in temperature, and smaller particles.

Density • Density equals mass divided by volume • Every material has a different density • No matter how small that sample of a material may be the density is the same. • Less dense objects float on more dense objects.

Physical/Chemical Changes • Physical Changes alters the appearance of an object, like size and shape • EX. Ripping paper • Chemical changes create a new substance. • EX. Burning paper • Energy is always added or released.

Physical/Chemical Properties • Physical properties describe an object with observations not inferences. • EX. The chair has 4 legs, not the chair is ugly. • Chemical properties describe the characteristics of a substance. • EX. Flammable

Conservation • The law of conservation of matter. • Matter can not be created or destroyed. • It can only be converted into a different substance.