MATTER AND ENERGY MATTER PART I CLASSIFYING MATTER





















- Slides: 29

MATTER AND ENERGY

MATTER PART I CLASSIFYING MATTER

is The Study of matter and how it changes Matter is anything having mass and volume. Mass- the amount of matter in an object Mass is measured with a balance, unit is grams • mass is related to weight but isn’t the same thing. • weight is dependent upon gravity. • mass never changes Volume- anything that takes up space, unit L, cm 3, m. L

IS AIR MATTER? Doestoair havesomass? YES, both, volume? air is matter 4

Composition- what matter is made of. Water is made of hydrogen and oxygen (H 2 O), tea has caffeine Properties-what matter is like. For example, water can dissolve many substances and caffeine is a stimulant. What caffeine is made of… What caffeine is like…

UEQ: In dealing with matter, how are composition, structure, properties and energy related? LEQ: What are the differences between physical and chemical properties? Extensive properties Intensive properties • Depends on the amount of matter present • Volume, mass, amount of energy in a substance • Does not depend on amount of matter present • Melting point, boiling point, density, ability to conduct electricity and transfer energy as heat

UEQ: In dealing with matter, how are composition, structure, properties and energy related? LEQ: What are the differences between physical and chemical properties? Physical changes • Change that does not change the identity or chemical makeup of the substance • Cutting, melting, drawing into wire, crushing, temperature and pressure changes Chemical changes • Substance changes into new substance b/c chemical bonds have been broken or made • Occurs on molecular level • Noticed by temperature change, smell/odor, bubbles (gas), rust formation • Reactants products

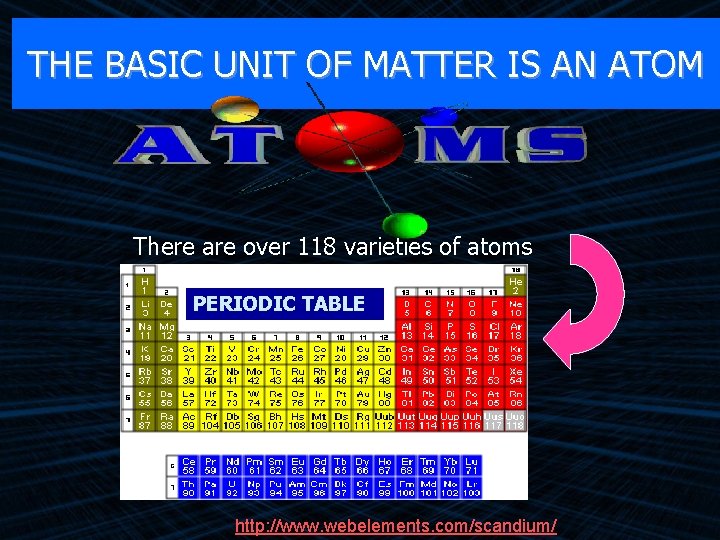
THE BASIC UNIT OF MATTER IS AN ATOM There are over 118 varieties of atoms PERIODIC TABLE http: //www. webelements. com/scandium/

ELEMENTS contain only one type of atom. Ex: hydrogen (H) is an element that contains only hydrogen atoms, carbon (C) contains only carbon atoms, oxygen (O) contains only oxygen atoms, etc. Atoms of elements can combine together to form compounds. COMPOUNDS are neutral groups of atoms held together by chemical bonds. Ex: CO 2, H 2 O, H 2, O 2

HOW DO WE CLASSIFY MATTER? All matter is classified as either a pure substance or a mixture Alloy rims/ mixture of Pure gold/Pure substance two metals 24 karat 18 karat End of introductory material. Return to index

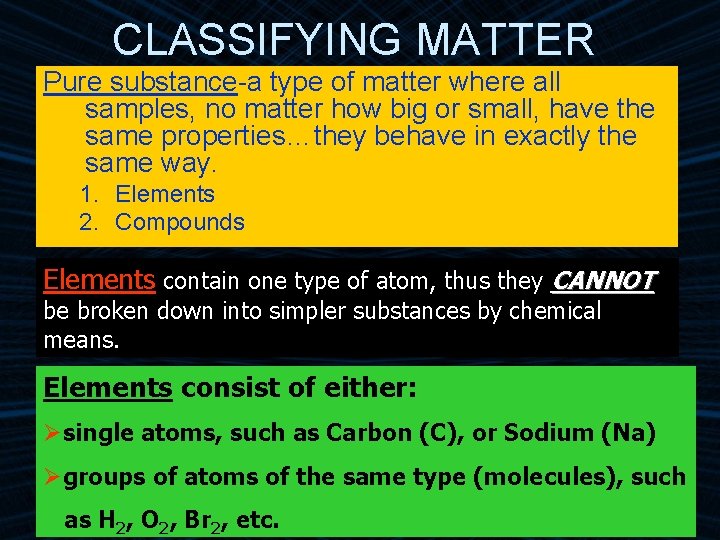
CLASSIFYING MATTER Pure substance-a type of matter where all samples, no matter how big or small, have the same properties…they behave in exactly the same way. 1. Elements 2. Compounds Elements contain one type of atom, thus they CANNOT be broken down into simpler substances by chemical means. Elements consist of either: Øsingle atoms, such as Carbon (C), or Sodium (Na) Øgroups of atoms of the same type (molecules), such as H 2, O 2, Br 2, etc.

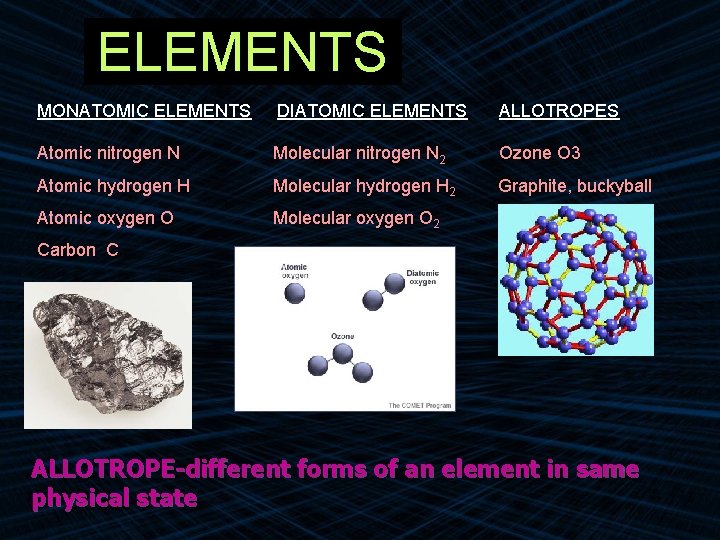
ELEMENTS MONATOMIC ELEMENTS DIATOMIC ELEMENTS ALLOTROPES Atomic nitrogen N Molecular nitrogen N 2 Ozone O 3 Atomic hydrogen H Molecular hydrogen H 2 Graphite, buckyball Atomic oxygen O Molecular oxygen O 2 Carbon C ALLOTROPE-different forms of an element in same physical state

COMPOUNDS Compounds are substances made up of 2 or more different elements that are chemically combined. CO 2, H 2 O, HCl, Na 2 SO 4 COMPOUNDS CAN BE BROKEN DOWN INTO SIMPLER SUBSTANCES BY CHEMICAL MEANS ONLY. COMPOUNDS HAVE DIFFERENT PROPERTIES THAN THE ELEMENTS THAT MAKE THEM UP. Click on the links below to watch videos of compounds broken down into the substances that make them up. Example-hydrolysis of water: 2 H 2 O 2 H 2 + O 2 • water breaks down into the hydrogen and oxygen gas which make it up. http: //www. all-science-fairprojects. com/science_fair_projects/38/819/5 db 524216341764 c 1438 c 1 f 760 fddff 8. html http: //earth 2 tech. com/2008/07/31/mit-solar-energy-storage-breakthrough/ Example-dehydration of sugar: C 12 H 11 O 22 12 C(s) + 11 H 2 O(g) -Sugar breaks down into carbon and water

ELEMENTS TO COMPOUND Sodium, Na, is a soft, shiny metal that can be cut with a butter knife. When in contact with moisture it will “explode”. COMBINED WITH… Chlorine gas, Cl 2, which is a green, poisonous gas once used as a chemical weapon in military operations. Will Form…

TABLE SALT! Sodium metal and chlorine gas, under the right conditions, undergo a chemical change and combine to become…. . 2 Na(s) + Cl 2(g) 2 Na. Cl The final compound has properties different from the elements that formed it.

1) We are studying CHEMISTRY, the study of ____and how it ____. 2) Matter is anything that has___ and takes up ___. 3) ___ are the basic building blocks of matter. 4) Matter can be classified as either a ___ or a ___. 5) Substances are either ___ or ___. Answers: 1. matter, changes 2. mass, space 3. atoms 4. pure substance, mixture 5. elements, compounds Return to index

THUS FAR WE HAVE REVIEWED MATTER AS PURE SUBSTANCES. NOW LETS DISCOVER MATTER AS MIXTURES MATTER PURE SUBSTANCE ELEMENT Carbon (C) COMPOUND H 2 O MIXTURE ?

MATTER AS MIXTURES Mixtures are combinations of 2 or more substances where each substance retains its individual properties. HOW ARE MIXTURES LIKE COMPOUNDS? HOW ARE MIXTURES DIFFERENT FROM COMPOUNDS? THEY ARE MADE FROM TWO OR MORE SUBSTANCES MIXTURES DO NOT FORM CHEMICAL BONDS.

Classifying mixtures There are two types of mixtures: 1) HOMOGENEOUS 2) HETEROGENEOUS

is …a mixture that is the same throughout. A homogeneous mixture has a composition and properties that are identical regardless of the sample Ways to identify a homogeneous mixture: Ø one phase Ø uniformly mixed Ø won’t settle out Ø small particles A SOLUTION is another name for a homogeneous mixture

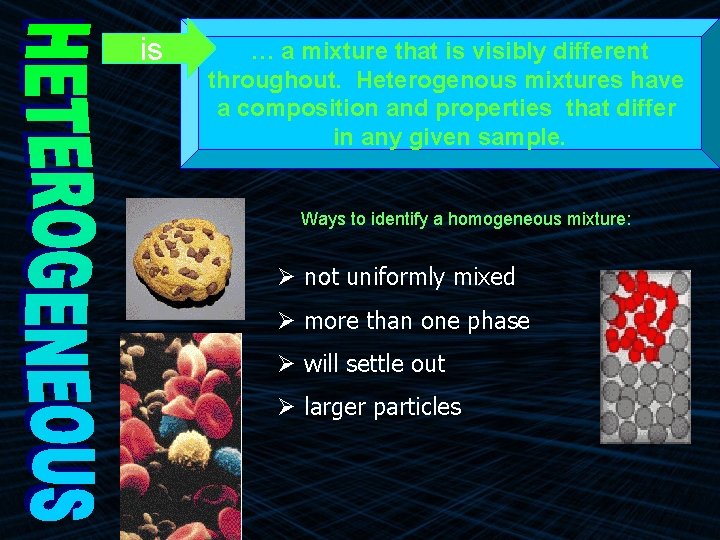
is … a mixture that is visibly different throughout. Heterogenous mixtures have a composition and properties that differ in any given sample. Ways to identify a homogeneous mixture: Ø not uniformly mixed Ø more than one phase Ø will settle out Ø larger particles

HETEROGENEOUS MIXTURES There are special types of heterogeneous mixtures: • Suspensions-appear uniform when mixed but settle out. – – Chocolate Quik Muddy water Pulpy oj Oil & Vinegar dressing • Colloids-appear uniform but they do not settle out. However, their particles are too large to be classified as a homogeneous solution. – – – Gelatin Milk Smoke Fog mayonnaise

TYNDALL EFFECT Colloids are evenly mixed, but their particles are too large to be considered homogeneous mixtures, which are also the same throughout. Colloids show the Tyndall Effect. The Tyndall effect is the scattering of light as a beam passes through a colloid. In each picture, the colloid on the left scatters the beam making it visible

Mixtures Summary Heterogeneous Mixture Homogeneous Mixture Not evenly mixed Evenly mixed Individual components retain their own properties Properties of combined components are usually different than those of each component Individual components can be easily seen Individual components can not be easily seen Can be easily separated Not as easily separated Ex. : salt & pepper mix, rocks, cereal, bag of assorted candy Ex. salt-water solution, tea, Cool -Aid drink

Practice Problems Determine whether each is a heterogeneous or homogeneous mixture: 1. 2. 3. 4. 5. Tossed salad Salt water Kool-aid Muddy water OJ with pulp Answers: 6. Tea 7. Banana nut bread 8. Pizza 9. Blood 10. Brass 1. He 2. Ho 3. Ho 4. He 5. He 6. Ho 7. He 8. He 9. He 10. Ho Return to index

Now, lets put it all together and determine how to tell substances (compounds and elements) from mixtures. • Pure Substances – Made of either elements or compounds that are chemically bonded. – Cannot be separated by physical means. – When combined they take on new properties different from the original elements. – Examples: • • Glucose: C 6 H 12 O 6 Table Salt: Na. Cl Oxygen Gas: O 2 Carbon Dioxide CO 2 • Mixtures: – Two or more substances mixed together but not chemically combined. • Tea = Crushed Leaf + H 2 O • Rocks = minerals + sediments + organic matter • Sugar Water = Sugar + H 2 O – Each component retains its own identity; it does not change into something else. – Can be separated by physical means – Examples: • Sweet tea • Trail mix • Air (O 2, N 2, CO 2, Ar)

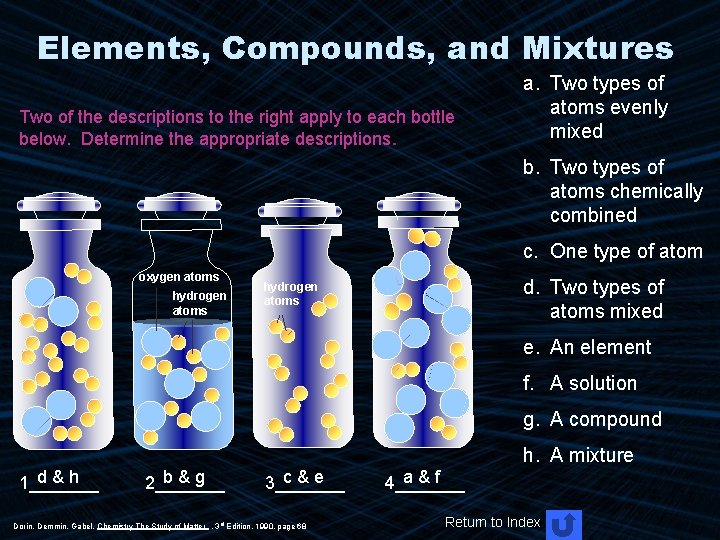
Elements, Compounds, and Mixtures Two of the descriptions to the right apply to each bottle below. Determine the appropriate descriptions. a. Two types of atoms evenly mixed b. Two types of atoms chemically combined c. One type of atom oxygen atoms hydrogen atoms d. Two types of atoms mixed hydrogen atoms e. An element f. A solution g. A compound h. A mixture d&h 1_______ b&g 2_______ c&e 3_______ Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 a&f 4_______ Return to Index

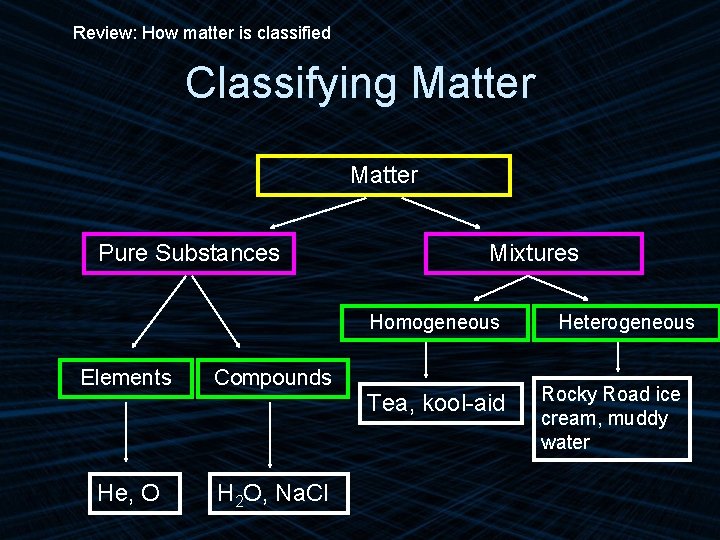
Review: How matter is classified Classifying Matter Pure Substances Mixtures Homogeneous Elements Compounds He, O H 2 O, Na. Cl Tea, kool-aid Heterogeneous Rocky Road ice cream, muddy water

More review Classify the following substances as: Pure substance, heterogeneous mixture, or homogeneous mixture Gatorade Homogenous mixture Fruit Loops Heterogeneous mixture Gasoline Pure substance Dirt Heterogeneous mixture Sugar Crystals Pure Substance Air Homogeneous mixture Granite Heterogeneous mixture Return to index