Properties of Matter Matter What is matter Matter










- Slides: 17

Properties of Matter

Matter What is matter? Matter is anything that has mass and takes up space. The particle model of matter shows that all forms of matter are made up of tiny particles that are in constant motion. Remember: Mass is the amount of matter in an object and is measured in Kg. Volume is the amount of space an object takes up and is measured in L or m 3. 55 ml = _____ cm 3 Formula? ? Volume = length x width x height

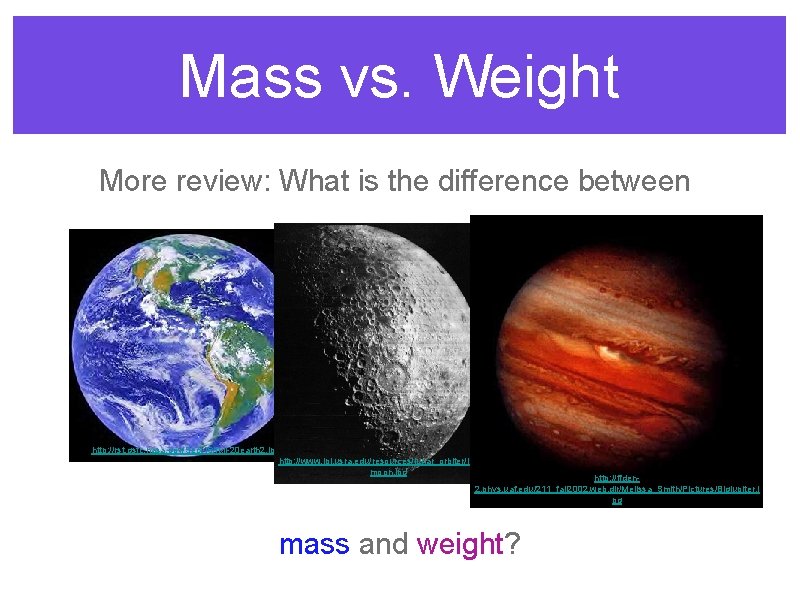
Mass vs. Weight More review: What is the difference between http: //rst. gsfc. nasa. gov/Sect 16/full-20 earth 2. jpg http: //www. lpi. usra. edu/resources/lunar_orbiter/images/ moon. jpg http: //ffden 2. phys. uaf. edu/211_fall 2002. web. dir/Melissa_Smith/Pictures/Bigjupiter. j pg mass and weight?

Inertia Is a property of matter. . . (Bill Nye the Science Guy) Race car and dump truck boulder and pebbles Intro to Newton’s first law: it’s harder to move a boulder than a pebble because of inertia just like it is harder to move a dump truck. But once the dump truck gets started moving it is harder to stop because of inertia. We’ll do more later.

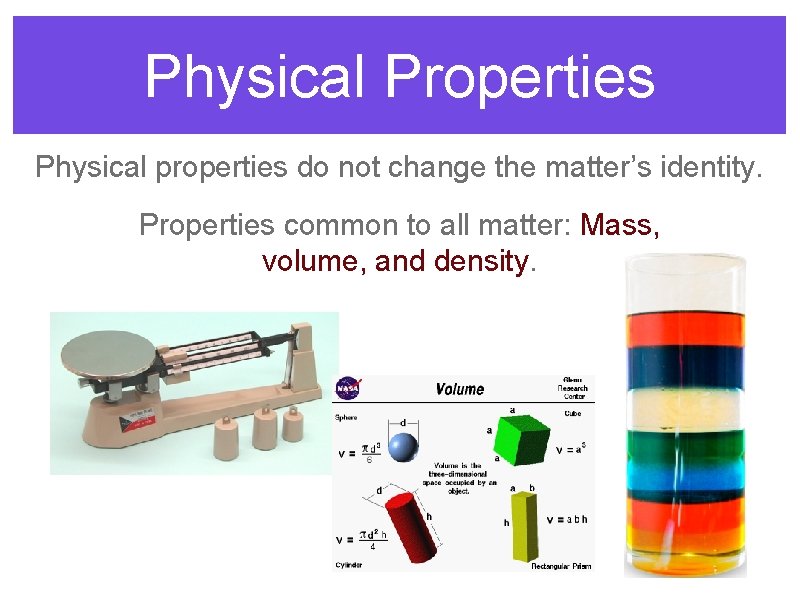
Physical Properties Physical properties do not change the matter’s identity. Properties common to all matter: Mass, volume, and density.

Other Physical Properties • Color • Size • Shape • Phase (solid, liquid, gas) • Luster (how shiny something is) • Ductility (pulled into wire) • Malleability (moldable) • Solubility (able to dissolve) • Conductivity (able to transfer heat or electricity)

What are the physical properties for the following? Iron Pyrite Milk Sugar Cubes Aluminum Foil Hot Cocoa Rocks Copper Wire Wooden cooking utensils

Chemical Properties Chemical properties describe matter’s ability to change into new matter with different properties. Reactivity: The ability of two or more substances to combine and form one or more new substances.

Flammability: the ability of a substance to burn.

Chemical Changes A chemical change is the process of one or more substances combining to form a new substance with different properties. Hydrogen gas + Oxygen gas = liquid water 2 H 2 + 02 = 2 H 20

Evidence of Chemical Change • Change in color or odor • Formation of a gas • Release or absorption of energy (sound, light, heat) • Formation of a precipitate (solid formed)

Physical Changes A physical change is the process in which matter changes in size, shape, or phase (state). A new substance is NOT made. The composition stays the same.

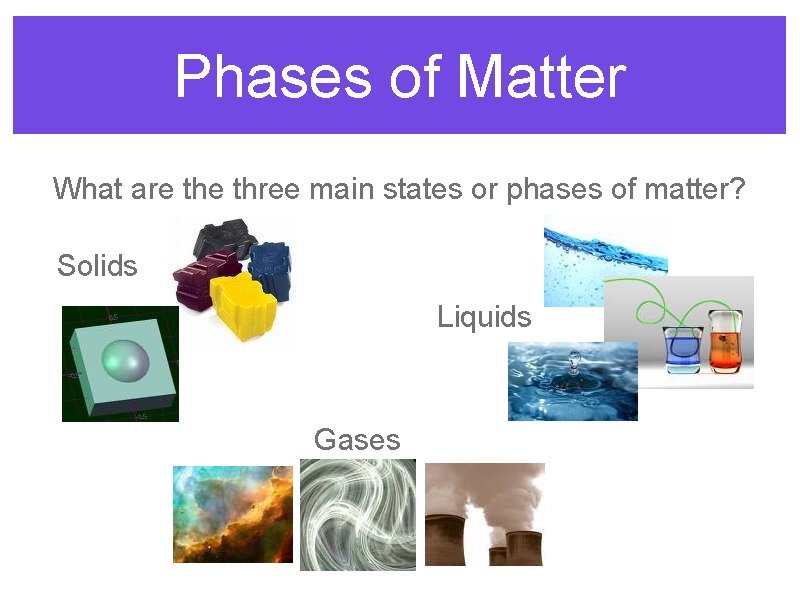
Phases of Matter What are three main states or phases of matter? Solids Liquids Gases

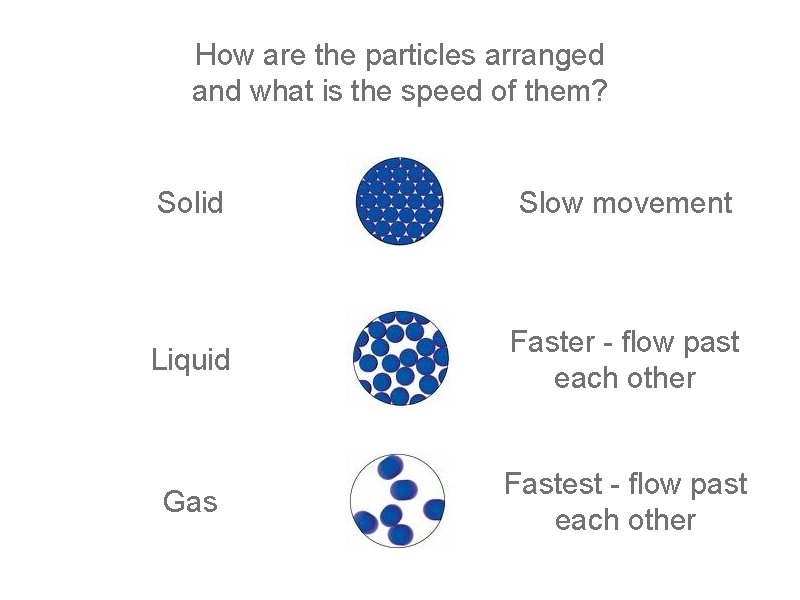
How are the particles arranged and what is the speed of them? Solid Slow movement Liquid Faster - flow past each other Gas Fastest - flow past each other

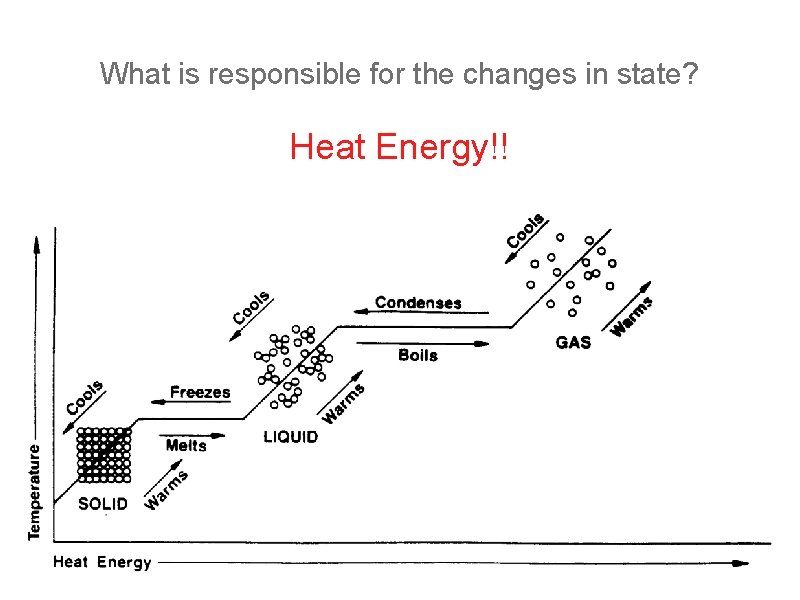
What is responsible for the changes in state? Heat Energy!!

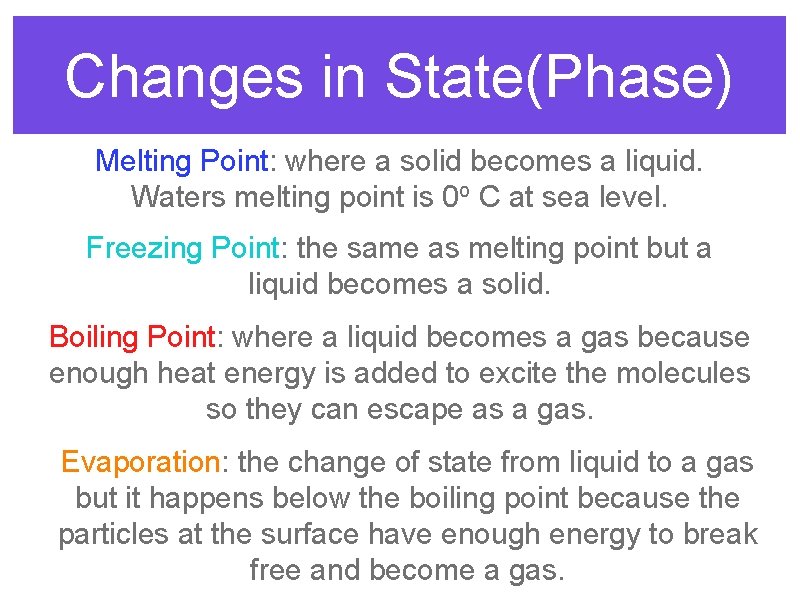
Changes in State(Phase) Melting Point: where a solid becomes a liquid. Waters melting point is 0 o C at sea level. Freezing Point: the same as melting point but a liquid becomes a solid. Boiling Point: where a liquid becomes a gas because enough heat energy is added to excite the molecules so they can escape as a gas. Evaporation: the change of state from liquid to a gas but it happens below the boiling point because the particles at the surface have enough energy to break free and become a gas.

Condensation: a change in state from a gas to a liquid. Sublimation: a change of state from a solid to a gas.
 Extensive vs intensive properties
Extensive vs intensive properties Physical properties of ice cube
Physical properties of ice cube Properties of matter definition
Properties of matter definition Properties of matter elasticity
Properties of matter elasticity Big idea 8 properties of matter
Big idea 8 properties of matter Objectives of properties of matter
Objectives of properties of matter Chapter 2 properties of matter
Chapter 2 properties of matter Properties of matter objectives
Properties of matter objectives Is odor observable or measurable
Is odor observable or measurable Strength property of matter
Strength property of matter Useful material at home
Useful material at home Properties of liquid in matter
Properties of liquid in matter What is matter grade 7
What is matter grade 7 Properties used to describe matter
Properties used to describe matter Matter and its properties
Matter and its properties Natural science grade 7 term 3
Natural science grade 7 term 3 Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 Common properties of matter
Common properties of matter
































