Materials Properties Electrical properties Magnetic properties Optical properties



- Slides: 27

Materials Properties • Electrical properties • Magnetic properties • Optical properties

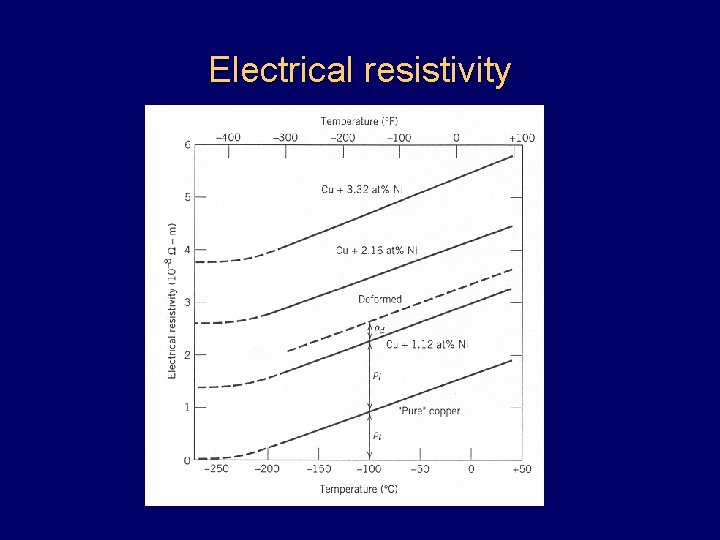
Electrical properties • • Ohm’s law Resistance, resistivity, conductivity • Matthiessen’s rule

Electrical resistivity

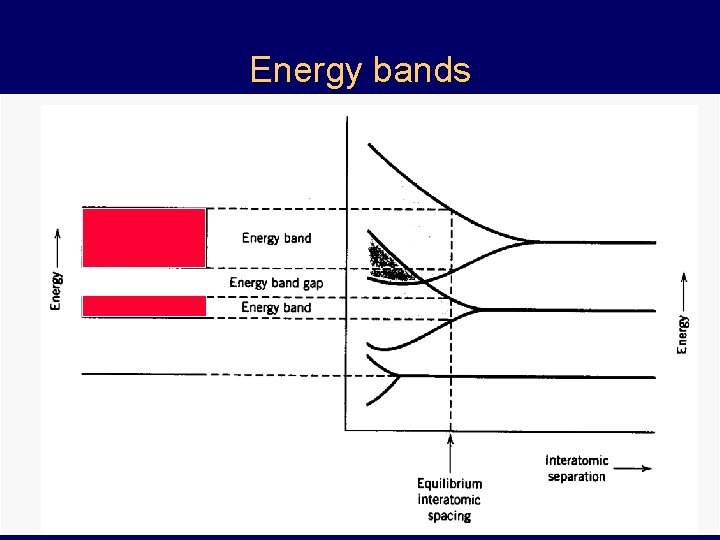
Energy bands discrete energy levels (Pauli exclusion principle) K L M splitting into energy bands (N=12)

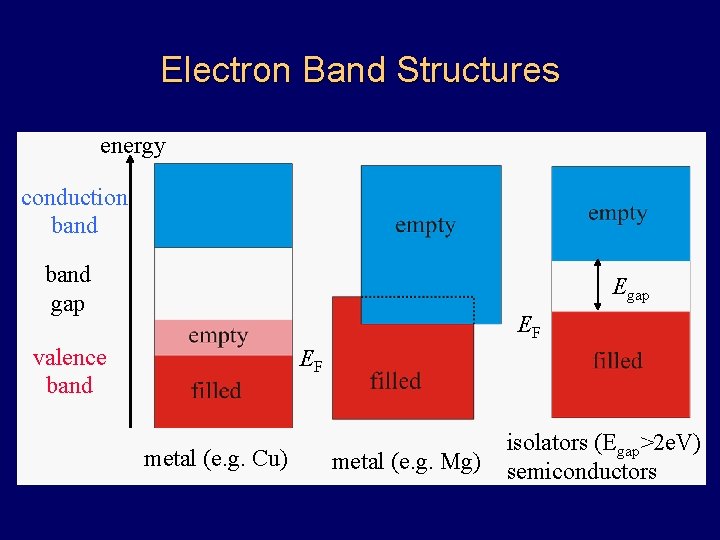
Electron Band Structures energy conduction band gap EF valence band EF metal (e. g. Cu) metal (e. g. Mg) isolators (Egap>2 e. V) semiconductors

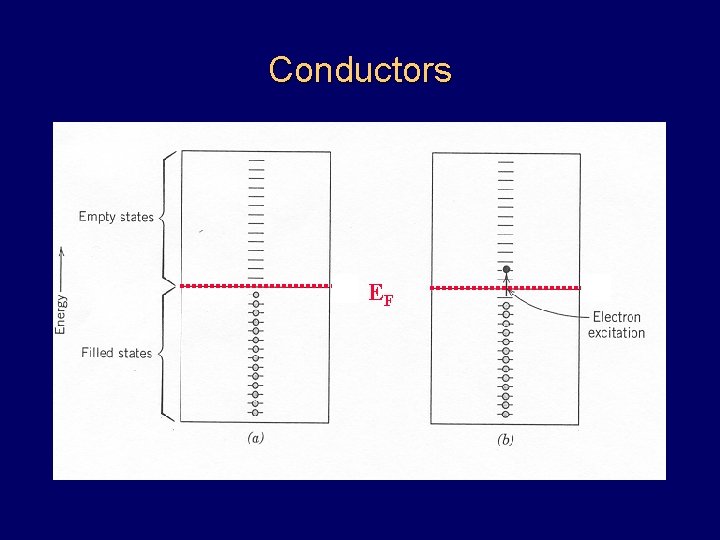
Conductors EF

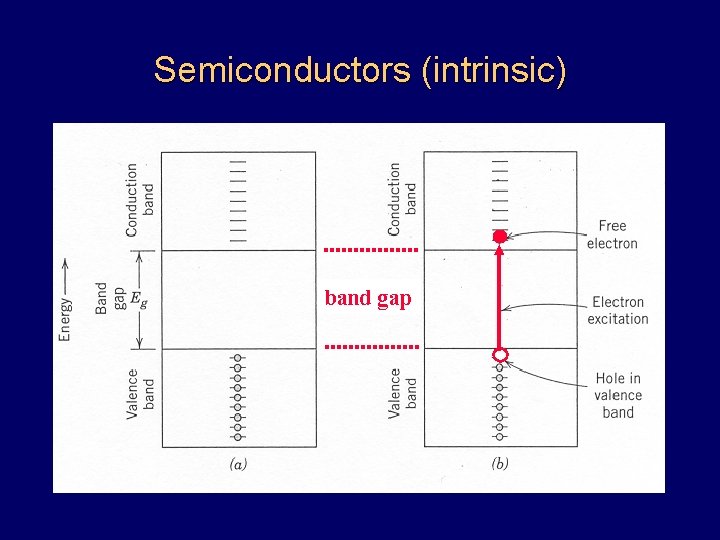
Semiconductors (intrinsic) band gap

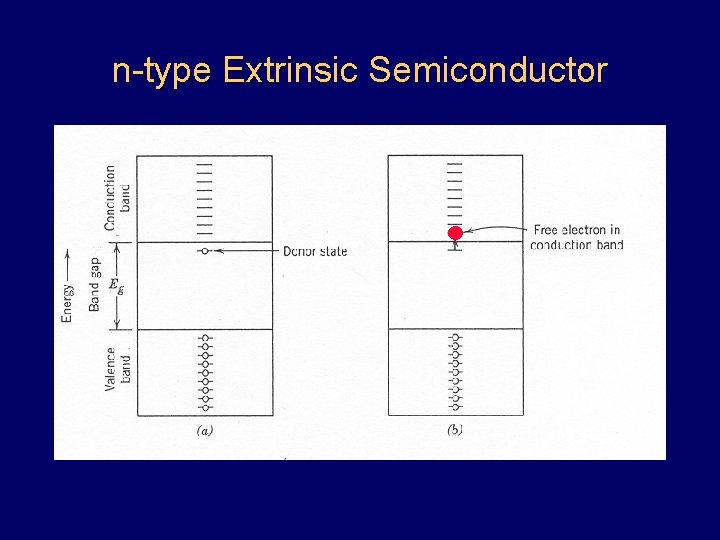
n-type Extrinsic Semiconductor

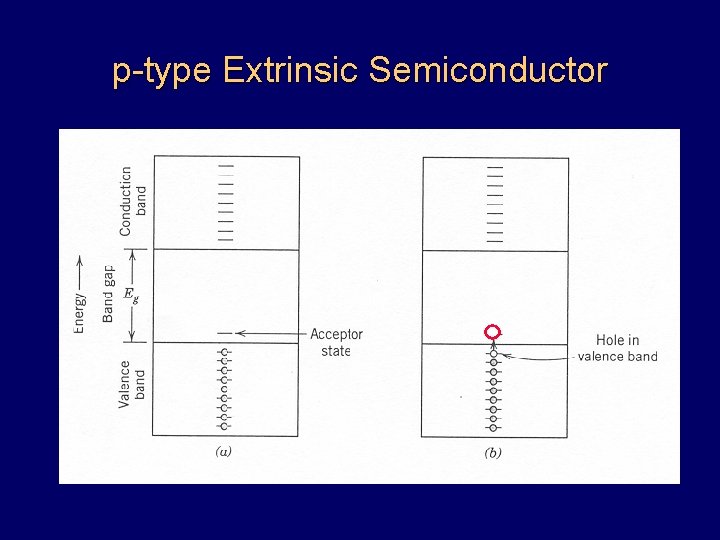
p-type Extrinsic Semiconductor

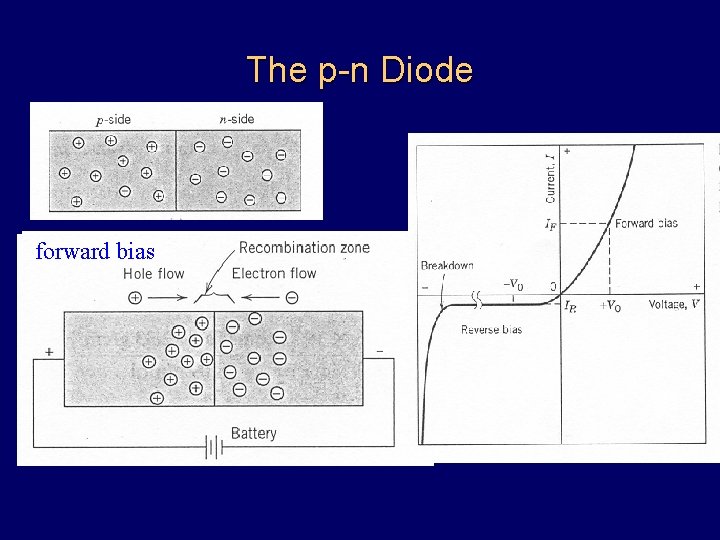
The p-n Diode reverse bias forward bias

Magnetic properties • Magnetic field strength, magnetic flux density, magnetization, permeability, and magnetic susceptibility

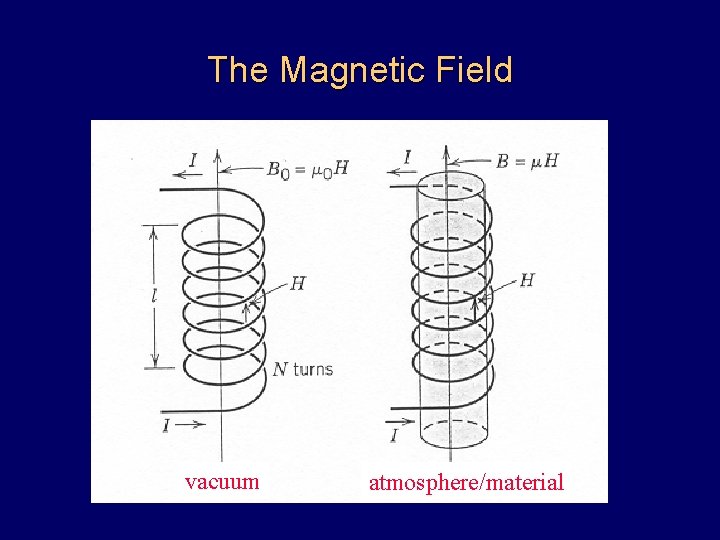
The Magnetic Field vacuum atmosphere/material

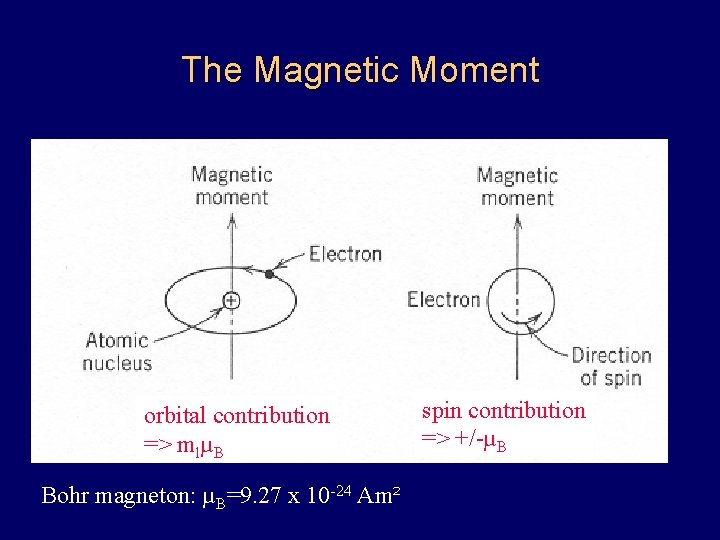
The Magnetic Moment orbital contribution => mlµB Bohr magneton: µB=9. 27 x 10 -24 Am² spin contribution => +/-µB

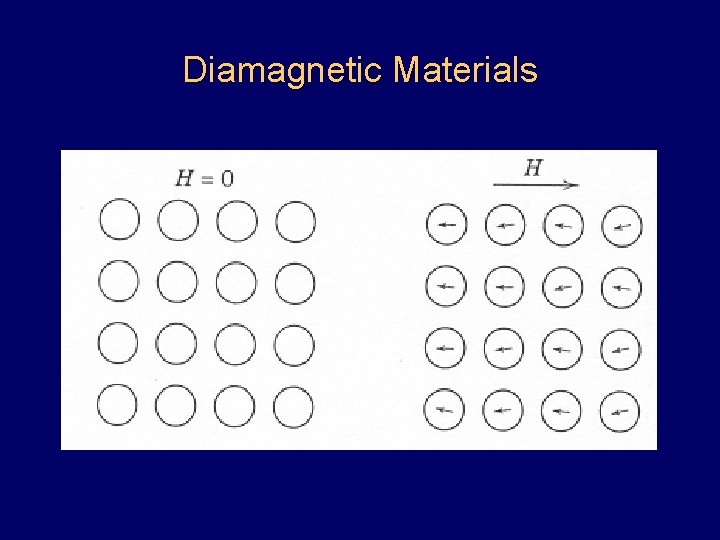
Diamagnetic Materials

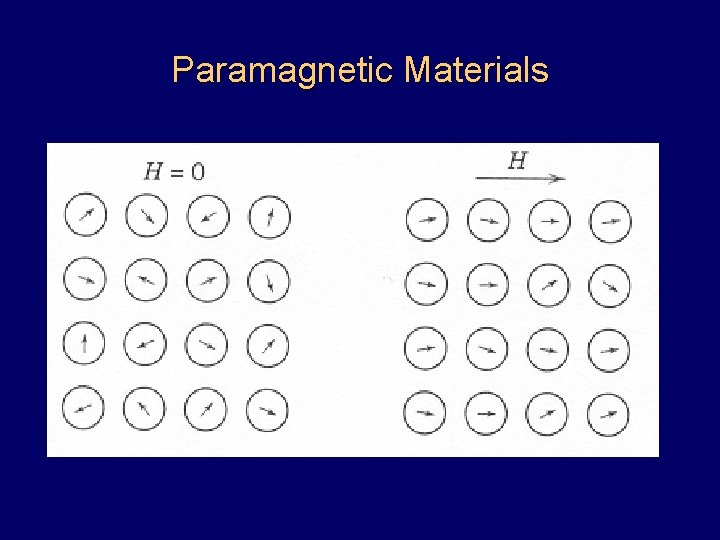
Paramagnetic Materials

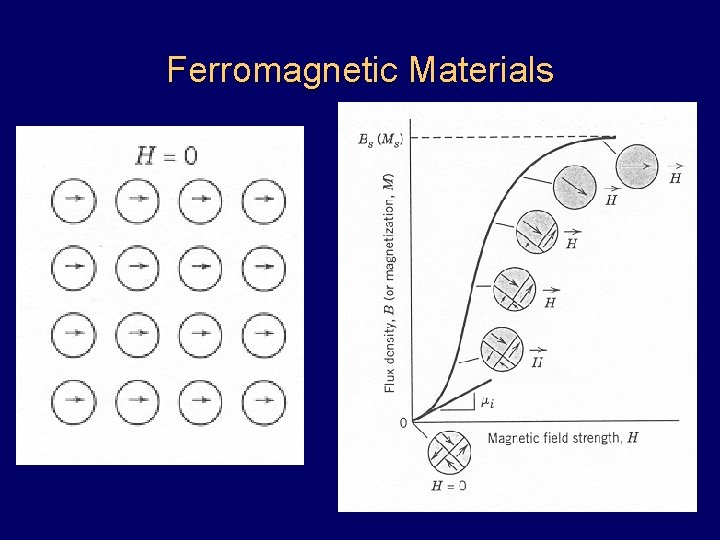
Ferromagnetic Materials

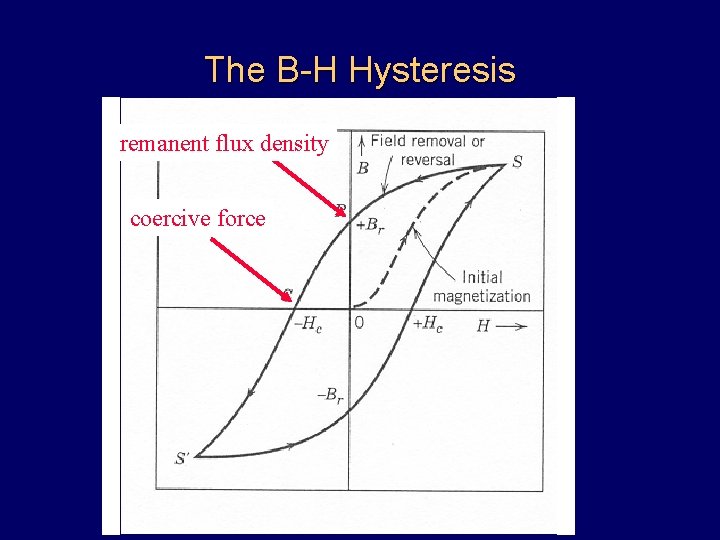
The B-H Hysteresis remanent flux density coercive force

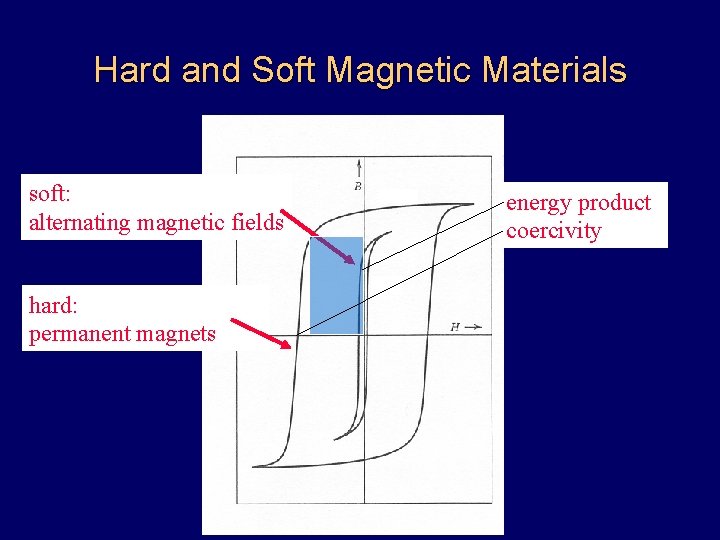
Hard and Soft Magnetic Materials soft: alternating magnetic fields hard: permanent magnets energy product coercivity

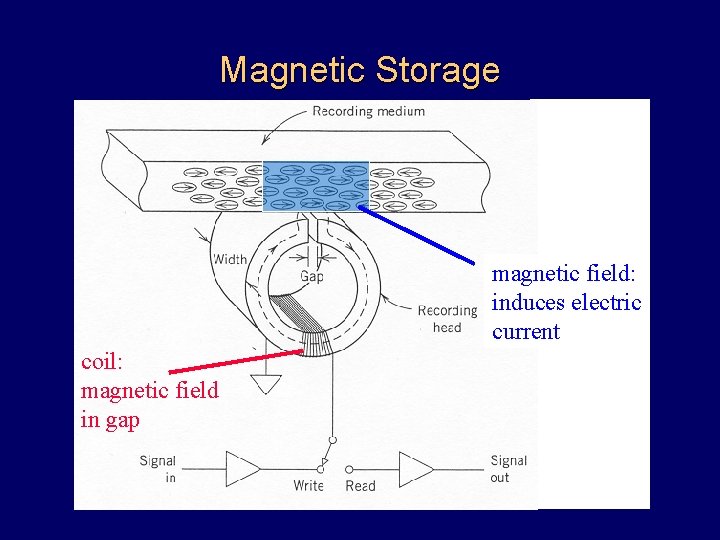
Magnetic Storage magnetic field: induces electric current coil: magnetic field in gap

Optical properties • Transmission • Refraction • Absorption

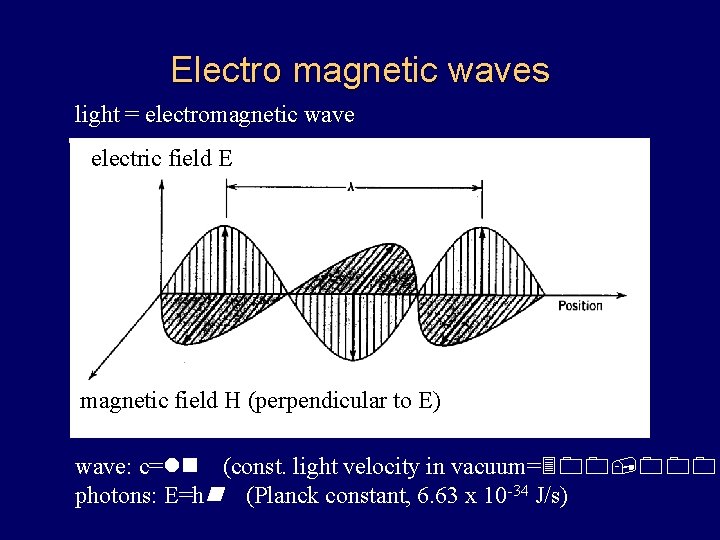
Electro magnetic waves light = electromagnetic wave electric field E magnetic field H (perpendicular to E) wave: c=ln (const. light velocity in vacuum=300, 000 photons: E=hn (Planck constant, 6. 63 x 10 -34 J/s)

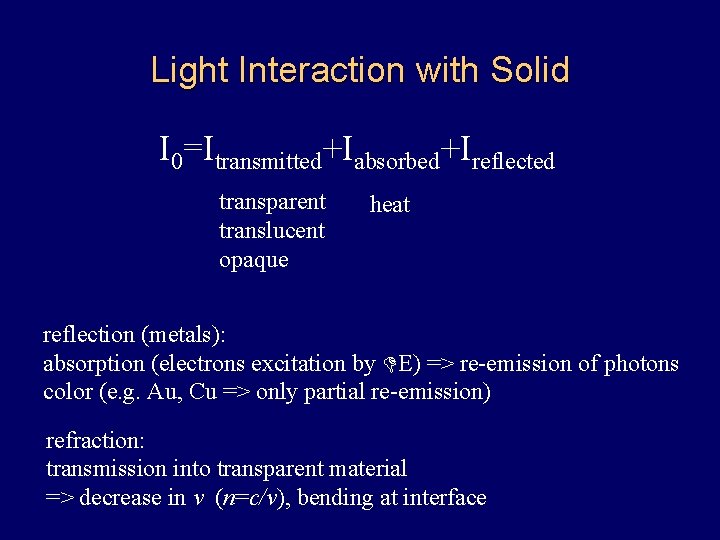
Light Interaction with Solid I 0=Itransmitted+Iabsorbed+Ireflected transparent translucent opaque heat reflection (metals): absorption (electrons excitation by DE) => re-emission of photons color (e. g. Au, Cu => only partial re-emission) refraction: transmission into transparent material => decrease in v (n=c/v), bending at interface

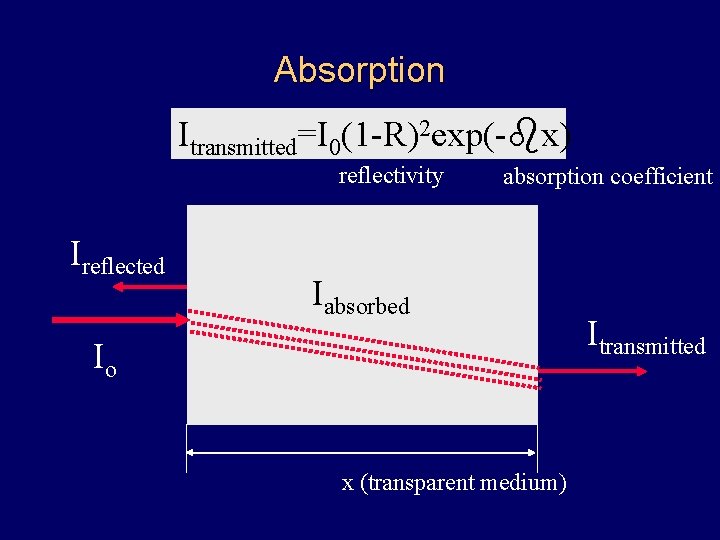
Absorption Itransmitted=I 0(1 -R)2 exp(-bx) reflectivity Ireflected absorption coefficient Iabsorbed Io x (transparent medium) Itransmitted

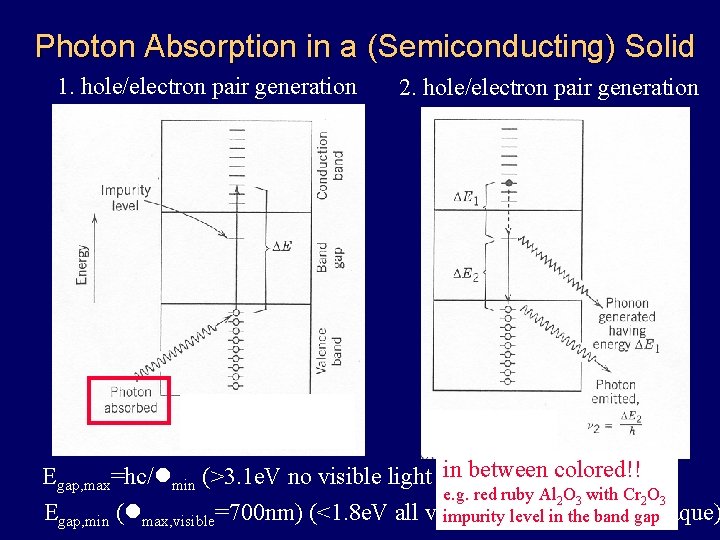
Photon Absorption in a (Semiconducting) Solid 1. hole/electron pair generation 2. hole/electron pair generation in between colored!! Egap, max=hc/lmin (>3. 1 e. V no visible light absorption=transparent) e. g. red ruby Al 2 O 3 with Cr 2 O 3 Egap, min (lmax, visible=700 nm) (<1. 8 e. V all visible light impurity levelabsorbed=opaque) in the band gap

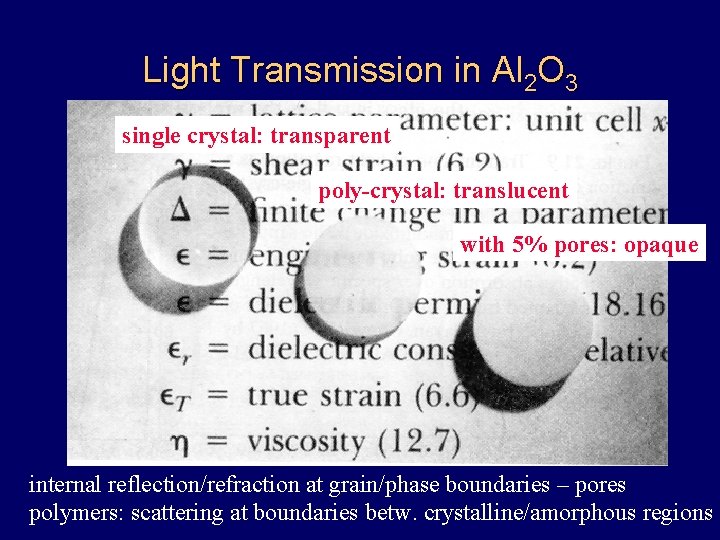
Light Transmission in Al 2 O 3 single crystal: transparent poly-crystal: translucent with 5% pores: opaque internal reflection/refraction at grain/phase boundaries – pores polymers: scattering at boundaries betw. crystalline/amorphous regions

Effects/Applications luminescence absorbing energy => re-emitting visible light (1. 8 e. V<hv<3. 1 e. V) fluorescence (<1 s) phosphorescence (>1 s) e. g. TV (fluoresc. coating) LED (forward bias diode – recombination=> light) photoconductivity illumination => generation of charge carriers e. g. light meters, solar cells optical fibres 1/0 impulses – high information density 24000 telephone calls by two wires e. g. 30000 kg Cu corresp. to 0. 1 kg high-purified Si. O 2 glass

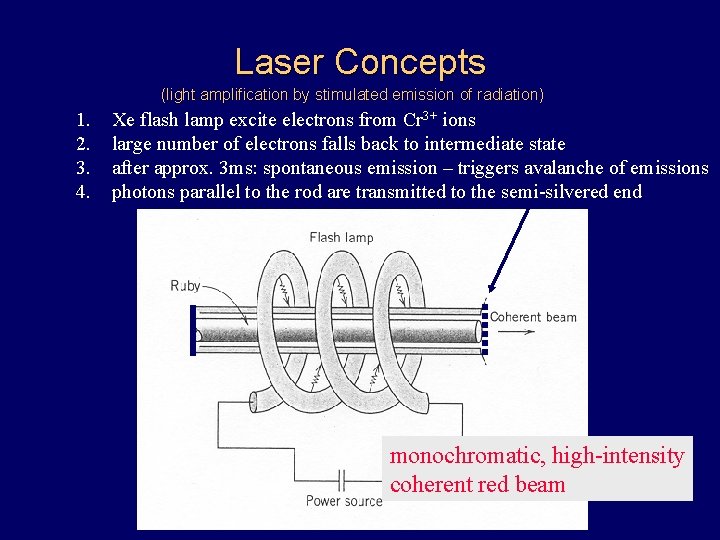
Laser Concepts (light amplification by stimulated emission of radiation) 1. 2. 3. 4. Xe flash lamp excite electrons from Cr 3+ ions large number of electrons falls back to intermediate state after approx. 3 ms: spontaneous emission – triggers avalanche of emissions photons parallel to the rod are transmitted to the semi-silvered end monochromatic, high-intensity coherent red beam